* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 13
Purified water wikipedia , lookup
Air well (condenser) wikipedia , lookup
Water purification wikipedia , lookup
Sewage treatment wikipedia , lookup
Portable water purification wikipedia , lookup
Water testing wikipedia , lookup
Ultraviolet germicidal irradiation wikipedia , lookup
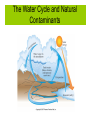
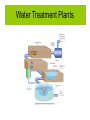
Chemistry for Changing Times 11th Edition Hill and Kolb Chapter 13 Chemistry John Singer Jackson Community College, Jackson, MI © 2007 Prentice Hall Water: Some Unique Properties Expands when it freezes. Water: Some Unique Properties High Heat of Vaporization Water has a very high heat of vaporization for a liquid. Therefore, a large amount of heat is required to vaporize a small amount of water. Water: Some Unique Properties High Specific Heat Specific heat is the amount of heat energy to necessary to raise the temperature of 1 gram of water 1 oC. Water, Water Everywhere 75% of the Earth’s surface is covered with water. Nearly 98% of that is seawater. The Water Cycle and Natural Contaminants The Water Cycle and Natural Contaminants Natural contaminants in water include; Gases including as radon, nonmetal oxides, and others. Dissolved minerals including cations and anions. Calcium, magnesium, and iron salts cause hard water. Organic Matter Bacteria, microorganisms, and animal wastes are all potential contaminants of natural waters. Chemical and Biological Contamination Waterborne Disease Contamination of water by pathogenic organisms was a serious problem. The EPA estimates that 30 million people in the U.S. are threatened by bacterial contamination of water supplies. It is estimated that 80% of all illness in the world is caused by water contamination. Chemical and Biological Contamination Acid Rain Sulfur and nitrogen oxides (SOx and NOx) are deposited as acid rain. Acidic precipitation damages the environment by lowering the pH of soil and lakes and streams. Acid rain also can corrode metals and dissolve limestone and marble. Chemical and Biological Contamination Sewage and Dying Lakes The release of sewage into waterways increases the biochemical oxygen demand (BOD), and leads to eutrophication (aging) of a lake. Organic matter can undergo either aerobic and anaerobic decay. Chemical and Biological Contamination The Water Cycle and Natural Contaminants Sewage and Dying Lakes Eutrophication is a natural process that is accelerated by the presence of human waste and runoff from farms, lawns, and other human activity. Industrial Pollution Manufacturing processes produce waste products and use water resources. Groundwater Contamination Approximately one-half of the U.S. population gets its drinking water from groundwater sources. Groundwater sources in many parts of the country are contaminated. Groundwater is easy to contaminate and difficult as well as expensive to clean up. Groundwater Contamination Nitrates Nitrate contamination of groundwater is particularly a problem in rural areas. Agricultural activity contributes fertilizers and animal wastes to water sources. Nitrates are very soluble. They are therefore difficult to remove from water supplies. Nitrates are a problem with infants usually less than one year old. They metabolize nitrate to nitrite. Nitrite ions then complex heme and the baby can turn blue and die. This condition is known as methemoglobinemia (blue baby syndrome). Groundwater Contamination Nitrates Groundwater Contamination Volatile Organic Chemicals (VOCs) VOCs can contaminate groundwater and add undesirable odor to drinking water. Also, many are carcinogenic. Sources include: industrial activity, oil and brine wells, landfills, leaking underground storage tanks, and illegal dumping of organic wastes. Making Water Fit to Drink More than 170,000 public water systems exist in the United States. The per capita use of water in the U.S. is almost 2 million liters per year. This includes water used for industrial, agricultural, and personal purposes. This use exceeds the per capital use of other nations. Making Water Fit to Drink The United States Safe Drinking Water Act was first passed in 1974. It was amended in 1986 and 1996. The Act authorizes the EPA to set, monitor, and enforce national health-based standards for contaminants in municipal water supplies. Making Water Fit to Drink The United States Safe Drinking Water Act Making Water Fit to Drink Parts per Million (ppm) 1 ppm = 1 g solute 106 g solution Parts per Billion (ppb) 1 ppm = 1 g solute 109 g solution Water Treatment Plants In most urban areas, water is treated at a water treatment plant before it is distributed to homes for consumption. Water Treatment Plants The first step in water treatment is to add slaked lime and alum to the water : The slaked lime and alum form the gelatinous aluminum hydroxide which coagulates colloidal particles with bacteria. These are then removed by filtering through sand and gravel filters. Charcoal is often present in the filtering process to remove odors and the water is aerated to improve taste. Water Treatment Plants Chemical Disinfection Chlorine is added to kill any remaining bacteria. Municipal drinking water often contains residual chlorine so that the water can be free from bacteria at any point in the distribution system. Ozone can also be used for bacterial disinfection and has the added advantage of killing many viruses. Water Treatment Plants Water Treatment Plants Other Technologies Ultraviolet light (UV) can also be used to disinfect water. It is most effective in small scale applications. One disadvantage is that it does not offer the residual protection that chlorine or ozone does. Water Treatment Plants Fluorides Many municipal water supplies have fluoride added to help prevent tooth decay. Tooth enamel is composed of a calcium phosphate complex called hydroxyapatite. Fluoride ions replace some of the hydroxide ions making the enamel harder and less affected by acids: Water Treatment Plants Fluorides Water is fluoridated by adding H2SiF6 or Na2SiF6 to a concentration 0.7-1.0 ppm. Early studies showed a 50 to 70% reduction in dental caries (cavities) in populations using fluoridated drinking water. Fluoridation of drinking water is not without controversy. Some people object to the fluoridation of drinking water. From Wastewater to Drinking Water Before wastewater can be returned to the environment, it should be treated to remove harmful contaminants. Municipal wastewater treatment is considered to involve up to three levels of processing. From Wastewater to Drinking Water Primary sewage treatment involves holding the sewage in settling ponds to allow heavier solids to precipitate out as sludge. From Wastewater to Drinking Water Secondary sewage treatment involves passing the effluent from the primary treatment through sand and gravel filters. During this process, aerobic bacteria can break down much of the organic matter. From Wastewater to Drinking Water Another form of secondary treatment is called the activated sludge method. The sewage is placed into tanks and aerated with large blowers. From Wastewater to Drinking Water Tertiary treatment involves further treating the sewage, such as charcoal filtration to absorb organic molecules, reverse osmosis, further filtration, distillation, etc. From Wastewater to Drinking Water The Newest Soft Drink: Bottled Water Bottled water is the fastest growing and most profitable segment of the beverage industry. Per capita consumption is 90 liters per year and growing. Many people think that drinking bottled water is better for one’s health than drinking tap water. In many cases, bottled water is someone else's tap water. Alternative Sewage Treatment Systems Sludge from municipal sewage treatment systems can be used as fertilizer. A number of communities allow primary treatment in settling ponds. The effluent is then allowed to flow into marshes that filter the sewage and use the nutrients. Toilets have been developed that compost wastes. Composting toilets use no energy or water. We Are the Solution to Water Pollution Water is essential to our quality of life. We must do what we can to maintain the quality of our water sources.