* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Risk of HIT
Survey
Document related concepts
Transcript
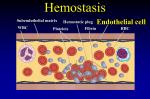
Heparin-Induced Thrombocytopenia Farzaneh Dastan Assistant Professor of Pharmacotherapy, Pharm D, BCPS SBMU Case A 64-year-old woman Hospitalized with endocarditis Condition is clinically stable while she is receiving intravenous antibiotic agents Decrease in platelet count from 161,000 per cubic millimeter on day 7 of hospitalization to 60,000 per cubic millimeter on day Receiving low-molecular weight heparin at a dose of 40 mg per day since admission. How should her case be further evaluated and treated? Epidemiology 1 in 5000 hospitalized patients Incidence rates of 1 to 3%: reported after cardiac surgery. Thromboembolic complications: 50% of patients with confirmed HIT. Most frequent complications: Venous thrombosis of the large vessels of the lower limbs and pulmonary embolism followed by peripheral arterial thrombosis and then stroke; Myocardial infarction: uncommon Thrombotic complications may also affect other vessels, including the cerebral sinus or splanchnic veins. Heparin-associated Thrombocytopenia (HAT) Nonimmune heparin-associated thrombocytopenia Traditionally called type I HIT Mediated by direct interaction between heparin and circulating platelets, causing platelet clumping or sequestration. Self-limiting thrombocytopenia Affects 10% of patients receiving heparin Within the first 72 hours after heparin administration, Platelet counts do not drop below 100,000/mm Normalizes once the heparin is ceased Heparin Induced Thrombocytopenia Immediate Onset who received within the previous 90 days (especially, ≤30 days), persistent circulating (PF4)– heparin antibodies start abruptly on reexposure to heparin (rapidonset HIT) anaphylactoid reaction within 30 minutes after a heparin bolus Delayed onset Develops or worsens after heparin has been discontinued. These patients can present with thrombosis up to 3 weeks after heparin exposure Spontaneous or autoimmune HIT Rare but often catastrophic form of HIT Develops in the absence of heparin exposure Most often after major surgery (especially knee replacement) or recent infection. In contrast to typical HIT, in which the platelet count increases within 2 to 5 days after the start of an alternative anticoagulant Autoimmune HIT may persist for weeks. HIT Occurs 5–10 days after receiving heparin Median platelet count between 50 and 80*109 L Dropped by 50% Platelet count starts to rise 2–3 days after discontinuing heparin Returns to normal within 4–10 days Antibody disappears within 2–3 months after cessation of heparin therapy HIT Criteria (a) thrombocytopenia (ie, a drop of the platelet count to <100 109/L or a drop of >50% from the patient’s baseline (b) exclusion of other causes of thrombocytopenia (such as cancer, sepsis or chemotherapy) (c) resolution of thrombocytopenia after cessation of heparin Strategies & Evidence Risk of HIT: Diagnosis Treatment Key interventions: Prompt cessation of heparin (if still being administered) Initiation of an alternative anticoagulant at a therapeutic dose Argatroban After discontinuation: aPTT returns to normal within 2 hours Standard starting dose is 2 microg/kg per minute by continuous intravenous infusion Aadjusted to maintain the aPTT at 1.5–3 times baseline and not to exceed 100 seconds Metabolized by the liver Reduced dose and adjustment in; impaired liver function, critically ill patients, or cardiac surgery. aPTT at 4-hour intervals after drug initiation or dose change Dose adjustment is not required in the presence of isolated renal impairment Bivalirudin 0.15–0.2 mg/kg per hour Adjusted to achieve an aPTT 1.5–2.5 times Doses of 0.14 mg/kg per hour in patients with hepatic dysfunction 0.03–0.05 mg/kg per hour in patients with renal or combined hepatic and renal dysfunction doses of 0.04–0.03 mg/kg per hour in those receiving continuous renal replacement therapy Lepirudin and desirudin Lepirudin: approved for use in HIT Desirudin: Data limited dose of either 15 or 30 mg subcutaneously every 12 hours suggested Danaparoid Analysis of outcomes: Rate of treatment success (platelet count recovery without new thrombosis and absence of major adverse events requiring drug cessation): higher than 90%. Renally metabolized Approved for treatment of HIT No longer marketed in the United Drug shortages have limited its availability Subcutaneously or intravenously Does not cross the placenta. Fondaparinux Long half-life allows once-daily dosing Monitored by the use of anti-factor Xa assay In pregnant women with HIT It is worth noting that HIT developed in a few cases with the use of fondaparinux Easier than argatroban to use outside the intensive care unit. Exacerbations of HIT reported infrequently small percentage have true in vivo and in vitro crossreactivity If manifestations of HIT worsen despite sufficient levels of anti–factor Xa activity, treatment should be switched (e.g., to argatroban) Non-vitamin K Antagonist Oral Anticoagulants (NOACs) Recommended by the European Society of Cardiology Orally using fixed dosing Need little attention regarding monitoring and drug– drug interactions Well proven in the management of venous and arterial thromboembolism Can be continued long term without conversion to warfarin, which needs platelet count recovery Short course of parenteral argatroban followed by NOACs for the treatment of patients with HIT is safe and effective NOACs RENAL REPLACEMENT THERAPY Argatroban and danaparoid recommended for patients with HIT who need renal replacement therapy Use of regional citrate is suggested for patients with a past history of HIT PREGNANCY For pregnant women with acute or subacute HIT: Danaparoid has been suggested lepirudin or fondaparinux may be considered if danaparoid not available Warfarin Vitamin K antagonists (e.g., warfarin and phenprocoumon) should not be given until HIT has abated Platelet count has increased to >150,000 per cubic millimeter at a stable plateau for 2 consecutive days Increase the risk of venous limb gangrene and limb loss by decreasing the level of protein C Overlap with an alternative anticoagulant is needed Warfarin should be slowly introduced at a dose of not more than 5 mg to avoid large loading doses. SWITCHING TO AN ORAL ANTICOAGULANT Avoidance of vitamin K antagonists (VKA) such as warfarin Can induce skin necrosis owing to rapid depletion of protein C Once HIT is confirmed, patient receiving VKA should be held and (INR) should be reversed by vitamin K. Platelet recovery to normal level is a required to initiate VKA Warfarin should be slowly introduced at a dose of not more than 5 mg to avoid large loading doses. DURATION OF ANTICOAGULATION FOR HIT Continued for 4–6 weeks in patients with confirmed isolated HIT For a minimum of 12 weeks in patients associated with thromboembolic events. IVIg High-dose intravenous immune globulin G Dose of 2 g per kilogram of body weight over a 2-day period By blocking platelet Fcγ receptors Limited data: may be an option (along with anticoagulation) in patients at high risk for thrombosis and bleeding (e.g., those who are pregnant or have sinus-vein thrombosis complicating HIT) or in patients who have autoimmune HIT In patients with a history of HIT who require cardiac surgery: Postponing surgery until platelet-activating anti– PF4–heparin antibodies disappear Using heparin intraoperatively Removal of platelet-activating anti–PF4–heparin antibodies by plasmapheresis, Bivalirudin is a compatible anticoagulant for cardiac surgery if platelet-activating anti–PF4– heparin antibodies are present Prophylactic platelet transfusions avoided Risk of bleeding is very low Increase the risk of thrombosis Guidelines ACCP and national European guidelines recommend the use of a scoring system Need for therapeutic-dose anticoagulation in cases of acute HIT ACCP: assessing patients at high risk (>1%) for HIT every 2 to 3 days between day 4 and day 14 THANKS FOR YOUR ATTENTION