* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Insect-Derived Cecropins Display Activity against
Marine microorganism wikipedia , lookup
Neonatal infection wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Molecular mimicry wikipedia , lookup
Thermal shift assay wikipedia , lookup
Human microbiota wikipedia , lookup
Infection control wikipedia , lookup
Bacterial cell structure wikipedia , lookup
Antimicrobial copper-alloy touch surfaces wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Disinfectant wikipedia , lookup
Virus quantification wikipedia , lookup
Bacterial morphological plasticity wikipedia , lookup
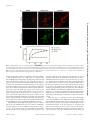
Insect-Derived Cecropins Display Activity against Acinetobacter baumannii in a Whole-Animal High-Throughput Caenorhabditis elegans Model Division of Infectious Diseases, Rhode Island Hospital, Alpert Medical School of Brown University, Providence, Rhode Island, USAa; Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USAb; Institute of Phytopathology and Applied Zoology, Justus-Liebig University, Giessen, Germanyc The rise of multidrug-resistant Acinetobacter baumannii and a concomitant decrease in antibiotic treatment options warrants a search for new classes of antibacterial agents. We have found that A. baumannii is pathogenic and lethal to the model host organism Caenorhabditis elegans and have exploited this phenomenon to develop an automated, high-throughput, high-content screening assay in liquid culture that can be used to identify novel antibiotics effective against A. baumannii. The screening assay involves coincubating C. elegans with A. baumannii in 384-well plates containing potential antibacterial compounds. At the end of the incubation period, worms are stained with a dye that stains only dead animals, and images are acquired using automated microscopy and then analyzed using an automated image analysis program. This robust assay yields a Z= factor consistently greater than 0.7. In a pilot experiment to test the efficacy of the assay, we screened a small custom library of synthetic antimicrobial peptides (AMPs) that were synthesized using publicly available sequence data and/or transcriptomic data from immune-challenged insects. We identified cecropin A and 14 other cecropin or cecropin-like peptides that were able to enhance C. elegans survival in the presence of A. baumannii. Interestingly, one particular hit, BR003-cecropin A, a cationic peptide synthesized by the mosquito Aedes aegypti, showed antibiotic activity against a panel of Gram-negative bacteria and exhibited a low MIC (5 g/ml) against A. baumannii. BR003-cecropin A causes membrane permeability in A. baumannii, which could be the underlying mechanism of its lethality. A cinetobacter baumannii is a Gram-negative, opportunistic bacterium that has recently emerged as a dangerous nosocomial pathogen (1–4). An increasing number of A. baumannii infections in patients have been detected among U.S. military service members injured in Iraq and Afghanistan (5). The genetic adaptability of A. baumannii allows it to gain resistance to a wide spectrum of commercial antibiotics, and the intrinsic presence of various efflux pumps in A. baumannii also contributes to an insensitivity to many antibiotics (6–8), resulting in very few viable treatment options for Acinetobacter infections (9–11). In addition, most of the clinical strains of A. baumannii also harbor a large antimicrobial resistance island (RI) of 86 kb that contains several beta-lactamase genes, conferring resistance to beta-lactam antibiotics (12, 13). The scarcity of antibiotics that can be used against A. baumannii infections drives the need for new kinds of antimicrobial agents (14). Empirical drug screening methods traditionally involve in vitro assays to measure the MICs for various pathogens. This is followed by in vivo testing of the drugs to measure their toxicity to eukaryotic cells (15). The disadvantage of these traditional assays is that a significant number of hit compounds show nonspecific toxicity to eukaryotic cells and are not promising as therapeutics (16). In this paper, we describe a whole-animal A. baumannii infection model compatible with large-scale compound screening using the model organism Caenorhabditis elegans. The nematode C. elegans has garnered interest among researchers as a model to study innate immunity as well as microbial pathogenesis due to its genetic tractability, transparency, small size, and conserved defense response pathways (17–20). In addition, the bacteriovorous C. elegans can be readily infected with a number of human pathogens and treated 1728 aac.asm.org with small molecules to evaluate curative and cytotoxic effects (21–24). To test the efficacy of the C. elegans-A. baumannii infection assay, we carried out a pilot screen of 68 insect-derived antimicrobial peptides (AMPs). AMPs are ubiquitously present in many cells and tissues of invertebrates, plants, and animals (25, 26). The physical properties of AMPs, including the presence of two or more positively charged amino acids and a large proportion of hydrophobic residues that fold into specific secondary structures with a certain amphipathicity, allow them to intercalate into and form pores in bacterial membranes, as well as to translocate inside bacterial cells (27). Moreover, AMPs also target the anionic phospholipid head groups in bacterial membranes by electrostatic interactions (26, 28, 29). These properties of AMPs that allow them to disrupt membrane architecture make it difficult for target or- Received 31 August 2014 Returned for modification 3 October 2014 Accepted 2 January 2015 Accepted manuscript posted online 12 January 2015 Citation Jayamani E, Rajamuthiah R, Larkins-Ford J, Fuchs BB, Conery AL, Vilcinskas A, Ausubel FM, Mylonakis E. 2015. Insect-derived cecropins display activity against Acinetobacter baumannii in a whole-animal high-throughput Caenorhabditis elegans model. Antimicrob Agents Chemother 59:1728 –1737. doi:10.1128/AAC.04198-14. Address correspondence to Eleftherios Mylonakis, [email protected]. Supplemental material for this article may be found at http://dx.doi.org/10.1128 /AAC.04198-14. Copyright © 2015, American Society for Microbiology. All Rights Reserved. doi:10.1128/AAC.04198-14 Antimicrobial Agents and Chemotherapy March 2015 Volume 59 Number 3 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital Elamparithi Jayamani,a,b Rajmohan Rajamuthiah,a,b Jonah Larkins-Ford,b Beth Burgwyn Fuchs,a,b Annie L. Conery,b Andreas Vilcinskas,c Frederick M. Ausubel,b Eleftherios Mylonakisa,b Antimicrobial Peptides against A. baumannii by HTS TABLE 1 Bacterial strains used in this study Source Reference(s) A. baumannii ATCC 19606 A. baumannii ATCC 17978 A. baumannii G5139 A. baumannii G5140 A. baumannii GC7945 A. baumannii GC7951 A. baylyi PS8004 Staphylococcus aureus MW2 P. aeruginosa PA14 E. coli OP50 Reference strain Reference strain Drain fluid Drain fluid adeB knockout adeB knockout Reference strain Clinical strain MW2 Clinical strain Lab 64, 76 64, 76 57 57 57 57 64 46 37 46 ganisms to develop resistance and make them novel candidates for new-drug development (25, 28, 30). In addition to potent antimicrobial activity, AMPs are also known to have immunomodulatory properties, which add to their potential as therapeutic agents (31, 32). In this pilot C. elegans-A. baumannii screen of 68 insect-derived AMPs, we identified 15 cecropin or cecropin-like peptides that prolonged the survival of worms infected with A. baumannii. The hit peptide BR003-cecropin A isolated from Aedes aegypti showed higher activity against A. baumannii than did the other cecropins and caused bacterial membrane perturbation. As a proof of concept, this small pilot screen of AMPs demonstrated that the automated, high-throughput C. elegans-A. baumannii screening assay can be used to screen small-molecule libraries to identify novel antimicrobials, which could lead to the identification of novel therapeutics for A. baumannii infections. Sytox orange, a dye that specifically stains dead worms, in M9 was added to each well using a Thermo Scientific multidrop Combi dispenser (Thermo Scientific, Waltham, MA) to obtain a final Sytox concentration of 0.7 M. The worms were then incubated overnight at 25°C. Stained plates were imaged with a Molecular Devices ImageXpress Micro (Molecular Devices, Sunnyvale, MA) automated microscope with bright-field and tetramethyl rhodamine isothiocyanate (TRITC) channels (Fig. 2 and 3) (16, 37). AMPs (2 mg/ml) dissolved in DMSO were screened at a final concentration of 2 g/ml and 1% DMSO in triplicate. In this screen, 2.5 g/ml polymyxin B (Fluka) in 1% DMSO and 1% DMSO were used as positive and negative controls, respectively. Image analysis was carried out using CellProfiler (http://www.cellprofiler .org/), a free, open-source image analysis software, using a series of image processing and analysis modules (16, 38). Survival was calculated by CellProfiler based on the ratio of Sytox-stained worm area to total worm area for each well of the assay plates. MATERIALS AND METHODS Bacterial strains, nematode strains, and culture conditions. All bacterial strains used in this study, shown in Table 1, were routinely cultured in Luria-Bertani broth (LB) or on LB agar at 37°C. The nematode strain glp-4 (bn2);sek-1 (km4) was used because the glp-4 mutation renders the nematodes incapable of producing progeny at 25°C (33) and because sek-1 mutant animals are relatively immunocompromised, thereby decreasing the duration of the assay (34–36). Infection assay. The assay was carried out in 384-well plates (Corning no. 3712) in a final volume of 70 l. Assay media consisted of 70% M9 buffer, 19% sheath solution (Union Biometrica no. 300-5101-000), 10% tryptic soy broth (TSB; Becton Dickson Co., NJ, USA), 10 M FeCl3,and 1% dimethyl sulfoxide (DMSO) or test compounds dissolved in 1% DMSO with a final density of bacteria at an optical density at 600 nm (OD600) of 0.03 (⬃5 ⫻ 106 CFU/ml). The assay workflow is outlined in Fig. 1. In brief, preparation of the bacterial culture started with an overnight culture of A. baumannii in LB broth that was spread on LB agar and incubated at 37°C for 8 h prior to infection assays. The bacteria were then scraped from the LB agar plates, pelleted, resuspended in M9 buffer containing 10% TSB (Becton Dickinson Co., NJ, USA) and 10 M FeCl3, and aliquoted into 384-well plates. Fifteen worms were then dispensed into each well of the assay plates with a complex object parametric analyzer and sorter (COPAS BioSort; Union Biometrica, Holliston, MA). Assay plates were sealed with Breathe-Easy membranes (Diversified Biotech, Dedham, MA) and incubated for 120 h with 80 to 85% humidity at 25°C. Subsequently, plates were washed 6 times with M9 medium using a BioTek ELx405 microplate washer (BioTek, Winooski, VT) to remove bacteria. All 15 worms were consistently recovered after each step in the washing process. After the last wash, ⬃25 l of assay medium with worms was left in each well. Before aspiration of the spent liquid, the plate was allowed to rest for 5 min, which is sufficient time to allow the worms to settle. Finally, 60 l of 0.9 M March 2015 Volume 59 Number 3 FIG 1 C. elegans-A. baumannii liquid killing assay workflow. Schematic representation of C. elegans-A. baumannii liquid infection assay for highthroughput screening. Antimicrobial Agents and Chemotherapy aac.asm.org 1729 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital Bacteria Jayamani et al. formed by coincubation of nematodes and bacteria in 10% TSB, 90% M9 with 10 M FeCl3 and either 1% DMSO (negative control) or 2 g/ml polymyxin B and 1% DMSO (positive control) and E. coli OP50 (positive control). The assay plate was incubated at 25°C for 5 days, and residual bacteria and debris were removed by washing followed by staining with Sytox orange. The images shown are TRITC fluorescent images of 6 wells in a column of a 384-well plate. (B) Optimization of the starting bacterial titer for the liquid killing assay. Z=-factor calculation. The quality of the HTS was evaluated by calculating the Z=-factor value from positive and negative-control data (39). Z= factor is calculated as 1 ⫺ [(3p ⫹ 3n)/(|p ⫺ n|)] where p and n are the standard deviations of the positive and negative controls, respectively, and p and n are the means of the positive and negative controls, respectively. The assay was validated with a Z= factor of ⬎0.5, which shows that the assay is consistent and robust enough for large-scale screens. The negative control was 1% DMSO, and the positive control was polymyxin B at a final concentration of 2.5 g/ml and 1% DMSO. Synthesis of insect-derived antimicrobial peptides. A total of 68 antimicrobial peptides (AMPs) from insects were synthesized based on publicly available sequence data or sequences generated by transcriptomic analyses of immune-challenged insects, such as the medicinal maggot Lucilia sericata, the red flour beetle Tribolium castaneum, the invasive harlequin ladybird Harmonia axyridis, and the greater wax moth Galleria mellonella (40–43). G. mellonella has been established as a powerful model host for human pathogens and a source for novel anti-infec- 1730 aac.asm.org RESULTS High-throughput C. elegans-A. baumannii liquid killing assay. We developed a C. elegans-A. baumannii liquid killing assay by testing several different media, bacterial concentrations, and incubation conditions. The nonpathogenic strain Escherichia coli OP50 (a widely used food source for laboratory-reared C. elegans) was used as a negative control. A medium containing 10% TSB and 90% M9 showed a good separation between the negative and positive controls, with 70% survival of worms incubated with E. Antimicrobial Agents and Chemotherapy March 2015 Volume 59 Number 3 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital FIG 2 C. elegans-A. baumannii liquid killing assay. (A) The assay was per- tives (44, 45). The 68 peptides (see the supplemental material) were produced by solid-phase synthesis and purified by reversed-phase chromatogaphy by GenScript (Piscataway, NJ, USA). The integrity of the peptides was confirmed by using liquid chromatography-mass spectrometry (LC/MS). Z score and hit identification. The hits were identified based on Z scores calculated from the CellProfiler survival ratio data. A Z score is defined as (x ⫺ )/, where x is the raw sample ratio score, is the mean for the population, and is the standard deviation for the population. Test samples with Z scores of ⬎1.5 were considered hits. Hit validation assay. The hit compounds that prolonged worm survival in the A. baumannii-C. elegans infection assay were tested at two different concentrations (5 g/ml and 10 g/ml) in triplicate (data not shown) (37, 46). MIC assay. The MIC assay was performed in 384-well plates in triplicate by the broth microdilution method adapted from an established protocol (47). The test compound range was set from 0.16 to 20 g/ml by performing 2-fold serial dilutions. The assay volume was 70 l containing M9 buffer, 10% TSB, and A. baumannii ATCC 19606 at an initial density corresponding to an OD600 of 0.03 (5 ⫻ 106 CFU/ml). DMSO (1%) and polymyxin B (2.5 g/ml in 1% DMSO) were used as negative and positive controls, respectively. The bacteria were incubated overnight at 37°C, and the bacterial growth was measured to determine antimicrobial activity of the test compounds. Disc diffusion assay. Mid-log-phase bacteria (50 l) at an OD600 of ⬃0.06 (1 ⫻ 107 CFU/ml) were plated onto LB agar plates and dried for 10 min to create a bacterial lawn. The discs were prepared by adding 10 l of 1 mg/ml stock solutions of cecropin A or polymyxin B in DMSO or DMSO as controls on 6-mm Whatman filter paper disks (Fisher Scientific). The discs were placed on the bacterial lawn and incubated overnight at 37°C, and the diameter of the zone of inhibition for each disc was measured. Each experiment was repeated three times (48). Membrane permeability assay. A. baumannii ATCC 19606 cultures were harvested at exponential growth phase, washed twice, and then resuspended in Hanks medium (catalog no. 14025-092; Life Technologies) supplemented with 10 mM D-glucose at a bacterial concentration corresponding to an OD600 of 0.06 (1 ⫻ 107 CFU/ml) and a final concentration of 1 M Sytox green. One hundred-microliter aliquots of this suspension were transferred to 96-well plates. Sytox green fluorescence (excitation wavelength [ex], 485 nm; emission wavelength [em], 520 nm) was measured in a fluorescence plate reader (BioTek microplate readers) (14, 49, 50). BRR003-cecropin A and polymyxin B were added at the corresponding 2⫻ MICs, and the fluorescence was monitored for 60 min at 10-min intervals. DMSO at 1% and 2.5 g/ml polymyxin B were used as negative and positive controls, respectively. The experiment was repeated three times. Fluorescence microscopy. A. baumannii was grown to mid-log phase at 37°C and treated with 5⫻ the MIC of cecropin A, the positive control (2 g/ml polymyxin B), or the negative control (1% DMSO). After 2 h of incubation at 30°C, the treated cultures were harvested and stained with 1 g/ ml FM4-64, 2 g/ ml DAPI, and 0.5 M Sytox green (Molecular Probes/Invitrogen). A 1-l portion of stained cells was transferred to a 1.2% agarose pad containing 20% LB medium for observation by using confocal laser microscopy (TCS NT; Leica Microsystems) (51, 52). Antimicrobial Peptides against A. baumannii by HTS elegans with or without 2 g/ml polymyxin B. Image analysis was carried out using CellProfiler; worms identified in the bright field are outlined in red, and dead worms identified in the Sytox orange fluorescent image are in green. (B) The Z= factor was calculated from survival percentage determined by CellProfiler analysis of positive- and negative-control images. The calculated Z= factor for the particular screening assay shown was 0.7. (C) The left half of the 384-well plate shows live worms incubated with A. baumannii in the presence of polymyxin B as a positive control, and the right half shows dead worms in the presence of DMSO as a negative control. coli OP50 and 30% survival with A. baumannii after 5 days (data not shown). Although iron acquisition mechanisms vary among Acinetobacter strains (53), recent studies by Eijkelkamp et al., Nwugo et al., and Dorsey et al. showed that the concentration of iron in the medium has a significant influence on Acinetobacter virulence factors (54–56). Therefore, we tested the addition of iron to the medium as a means of enhancing killing by A. baumannii ATCC 19606. Addition of 10 M FeCl3 to the assay medium increased killing efficiency in the liquid assay, decreasing worm survival to 5% (Fig. 2A). In addition, we found that the starting bacterial titer influenced the killing kinetics of worms. Worms treated with an initial concentration of A. baumannii ATCC 19606 or E. coli OP50 corresponding to an OD600 of 0.03 in medium supplemented with 10 M FeCl3 exhibited the maximum difference between survival on E. coli OP50 (80%) and A. baumannii (⬍3%) after 5 days incubation (Fig. 2B). Liquid killing assay quality assessment. The reproducibility and reliability of the liquid killing assay was evaluated by calculating the Z= factor from survival ratios based on CellProfiler analysis of bright-field and Sytox images using 1% DMSO and 2 g/ml polymyxin B as negative and positive controls, respectively (Fig. 3A and C). In 3 replicates, the Z= factor ranged from 0.6 to 0.7, March 2015 Volume 59 Number 3 confirming that the assay is robust and could be used for largescale screening of small-molecule libraries (Fig. 3B). Screening and identification of compounds effective against A. baumannii. The C. elegans-A. baumannii liquid killing assay was used to perform a pilot screen with a small library of insectderived antimicrobial peptides. The library includes 68 synthetic peptides belonging to different families of insect AMPs biosynthesized by a variety of different insect species. The AMP library was screened in triplicate at a final AMP concentration of 2 g/ml. We identified hits by calculating Z scores, i.e., the number of standard deviations by which a sample well deviated from a negative-control well, derived from the percentage survival data. The average Z score for the polymyxin B positive-control wells was 1.5, and we set that value as a threshold for “hit” wells. We identified 15 AMPs that prolonged worm survival upon exposure to A. baumannii that had a Z score of ⱖ1.5. Significantly, all of the hits were either cecropin (11) or cecropin-like (4) peptides (Table 2; also, see Fig. S1 in the supplemental material). In fact, all cecropins or cecropin-like peptides present in the peptide library were identified as hits. All the hit compounds were retested at 5 and 10 g/ml in the liquid killing assay and showed rescue at both concentrations, suggesting that even at relatively high concentrations, cecropins Antimicrobial Agents and Chemotherapy aac.asm.org 1731 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital FIG 3 Image data for the C. elegans-A. baumannii assay and Z= factor calculation. (A) Bright-field and Sytox orange-stained images of A. baumannii-infected C. Jayamani et al. TABLE 2 AMP hits in HTS assay based on Z scores of triplicates AMP Z1, Z2, Z3 Hyalophora cecropia Aedes aegypti Lucilia sericata contig 10925 Lucilia sericata contig 26125 Lucilia sericata contig 2999 Lucilia sericata contig 9468 Lucilia sericata contig 16625 Lucilia sericata contig 1380 Lucilia sericata contig 489 Lucilia sericata contig 1845 Lucilia sericata contig 21136 Sarcophaga peregrina Stomoxys calcitrans Lucilia sericata contig 25628 Lucilia sericata contig 25628 Cecropin A Cecropin A Cecropin Cecropin Cecropin Cecropin Cecropin Cecropin Cecropin Cecropin Cecropin Sarcotoxin IA Stomoxyn Stomoxyn start A Stomoxyn start G 1.8, 1.8, 1.8 1.7, 1.7, 1.6 1.8, 1.8, 1.8 1.7, 1.6, 1.7 1.6, 1.8, 1.8 1.7, 1.8, 1.8 1.8, 1.8, 1.8 1.8, 1.8, 1.8 1.8, 1.8, 1.7 1.7, 1.8, 1.6 1.7, 1.7, 1.6 1.8, 1.8, 1.7 1.8, 1.8, 1.8 1.8, 1.8, 1.7 1.8, 1.8, 1.8 are not toxic to worms (data not shown). To further address the toxicity of cecropin, we tested and compared the toxic effects of cecropins and polymyxin B by treating C. elegans with doses of increasing concentration of the drugs up to 10⫻ the MIC. The assay was carried out over 10 days with E. coli as the food source. In order to address the concerns that worms might starve due to the killing of the E. coli by the peptides, food was supplemented every day without drastically altering the assay volume. The assay was performed in triplicate with similar results. There was no drastic loss in viability for worms treated with cecropin A at a concentration of 10⫻ the MIC for 10 days. However, at a similar concentration, polymyxin B displayed significant toxicity toward worms (data not shown). Antibacterial activity of cecropin A and cecropin-like family. To determine if AMPs identified in the screen were acting directly on A. baumannii, we tested their ability to inhibit bacterial growth. We found that one particular hit peptide, BR003-cecropin A isolated from Aedes aegypti, had a very low MIC, ⬍5 g/ml (Table 3), and had activity against other Gram-negative bacteria as well, including E. coli and Pseudomonas aeruginosa, but not the Grampositive pathogen methicillin-resistant Staphylococcus aureus (MRSA) MW2 (Table 4). TABLE 3 MICs of cecropins and cecropin-like family of peptides Compound MIC (g/ml) Polymyxin B BR001 BR002 BR003 BR005 BR029 BR030 BR031 BR032 BR033 BR034 BR035 BR036 BR037 BR043 BR044 2.5 10 10 5 20 20 ⬎20 20 20 20 20 10 20 20 ⬎20 ⬎20 1732 aac.asm.org TABLE 4 In vitro antimicrobial activity of cecropin A MIC (g/ml) Compound Vancomycin Polymyxin B Gentamicin BR003-cecropin A E. coli (OP50) MRSA (MW2) P. aeruginosa (PA14) A. baumannii 1.2 2.25 0.6 3.5 75 0.6 10 4.5 An in vitro disc diffusion assay showed a clear zone of inhibition caused by discs containing BR003-cecropin A and polymyxin B (Fig. 4B). We further tested the activity of BR003-cecropin A on a panel of Acinetobacter species, including Acinetobacter baylyi and clinical strains of Acinetobacter baumannii such as A. baumannii G5139 and G5140, which overexpress the AdeABC efflux pump, and A. baumannii GC7945 and GC7951, which lack the AdeABC efflux pump (57). Interestingly, BR003-cecropin A displayed similar activities against all tested strains (Fig. 4A). Sequence analysis of cecropin A and cecropin like family from AMPs library. Sequence analysis of the cecropins that were hits in the screening assay showed the presence of tryptophan at the 1 or 2 position at the N terminus of many of the cecropins, although the most effective peptide BR003-cecropin A lacked the tryptophan (Fig. 5). The absence of tryptophan, as well as an increased number of positively charged amino acids in BR003-cecropin A may contribute to the low MIC of this cecropin against A. baumannii. Antimicrobial activity of cecropin A. The bactericidal efficiencies of cecropin A and other cationic peptides have been previously reported to be due to their ability to form pores in bacterial membranes (50, 58). We tested if treatment of A. baumannii A9844 with BR003-cecropin A caused increased membrane permeability by using fluorescence microscopy to observe the bacterial cells stained with the membrane dye FM4-64 and with Sytox green, a dye that penetrates only cells with compromised membranes (51). Bacteria treated with DMSO were short rods and showed membranes stained with FM4-64 but not Sytox green (Fig. 6A). Treatment with cecropin A caused the bacteria to be elongated and show bright Sytox staining (Fig. 6A). Interestingly, the positive-control polymyxin B showed a bacterial shape and fluorescence pattern similar to those of cecropin A (Fig. 6A). Maximum accumulation of Sytox green caused by cecropin A treatment was achieved at 10 g/ml, 2-fold above the MIC (data not shown). To evaluate the kinetics of membrane permeability, fluorometric measurements were taken at 10-min intervals for 60 min. Membrane permeability induced by cecropin A and polymyxin B was observed to be time dependent, suggesting that cecropin A rapidly kills bacteria by disrupting membrane integrity (Fig. 6B). DISCUSSION Drug-resistant A. baumannii is a National Institute of Allergy and Infectious Diseases (NIAID) category C pathogen and is also a member of ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.), a group of bacteria thought to be the most dangerous multidrug-resistant (MDR) microbes (59– 61). Treatment options are limited due to the variety of resistance traits harbored by these pathogens, including a spectrum of -lac- Antimicrobial Agents and Chemotherapy March 2015 Volume 59 Number 3 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital Source Antimicrobial Peptides against A. baumannii by HTS tamases, carbapenemases, aminoglycoside modification enzymes, and modifications in the penicillin-binding protein and outer membrane proteins (2, 10). The evolution of resistance to most conventional antibiotics within 5 years of their introduction has been a major threat to human health (11, 29), and polymyxin B and colistin are often treatments of last resort (9, 62, 63). Previous studies have used C. elegans as a model host to study the interaction of A. baumannii and Candida albicans by coinfec- FIG 5 Amino acid sequence analysis of cecropin A and cecropin-like peptides. The color scale highlights the level of conservation of specific amino acid residues. The distributions of certain hydrophilic and hydrophobic amino acids are conserved in all cecropins and cecropin-like peptides and are characteristic of cationic peptides. March 2015 Volume 59 Number 3 Antimicrobial Agents and Chemotherapy aac.asm.org 1733 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital FIG 4 Antimicrobial activity of cecropin A against A. baumannii. (A) MIC of BR003-cecropin A compared with that of polymyxin B with a panel of Acinetobacter strains. (B) Disc diffusion assay to measure zones of inhibition due to bactericidal activity of BR003-cecropin A against A. baumannii. The discs were prepared by adding 10 l of 1-mg/ml stock solutions of cecropin A or polymyxin B in DMSO by using DMSO alone as a control. Jayamani et al. tion in worms. These studies revealed that A. baumannii inhibits C. albicans filamentation, a key component of C. albicans virulence, highlighting the importance of pathogenic prokaryotic and eukaryotic interaction in the host (64). Using an agar-based C. elegans assay to evaluate an insertion mutant library of A. baumannii, Peleg et al. and Smith et al. identified many genes encoding A. baumannii virulence factors (64–66). Although these agar-based assays are useful for studying virulence factors of pathogenic bacteria and to carry out small-scale genetic screens, they are not appropriate for large-scale small molecule screens. In order to carry out large-scale chemical screens, we developed a C. elegans-A. baumannii liquid infection assay that could be used to screen large small-molecule libraries to identify new drugs that may be useful in treating A. baumannii infections. A key feature of our C. elegans assay is that it is able to assess antimicrobial activity of a small molecule while concurrently evaluating its toxicity. Liquid killing assays for high-throughput screens in C. elegans have been well established for Enterococcus faecalis, methicillinresistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa (16, 37, 46). The C. elegans-E. faecalis infection assay utilizes worms preinfected with a pathogen followed by treatment. The 1734 aac.asm.org rationale is that E. faecalis persistently colonizes the C. elegans intestine, which allows screening for compounds that cure an established infection. In contrast to E. faecalis, however, P. aeruginosa, MRSA, and A. baumannii do not form persistent infections in C. elegans. Moreover, in the case of P. aeruginosa, MRSA, and A. baumannii, preinfection of the worms with the pathogen cannot be used due to the high risk of contamination of the equipment during the dispensing of preinfected worms into 384-well plates and the difficulties of disinfecting equipment after each use (67). Therefore, we developed an automated, high-throughput liquid killing assay of C. elegans mediated by A. baumannii that involves dispensing worms into plates containing A. baumannii and compound. Using this assay, we performed a pilot proof-of-principle screen of a small library of 68 AMPs. Due to the fact that AMPs exploit fundamental conserved features of the bacterial cell wall, they are able to act against a broad spectrum of pathogens, including Salmonella enterica, E. coli, K. pneumoniae, P. aeruginosa, and Listeria monocytogenes (58, 68, 69). Also, because of the mechanism of action of AMPs, the incidence of bacterial resistance is very low. Moreover, they also exhibit anti-endotoxic effects and Antimicrobial Agents and Chemotherapy March 2015 Volume 59 Number 3 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital FIG 6 Cell permeability assay. (A) A. baumannii cells treated with BR003-cecropin A and polymyxin B stained with the red membrane dye FM4-64 and the membrane-impermeative dye Sytox green. Treatment with DMSO caused cells to be short rods stained only with FM4-64, whereas treatment with either cecropin A or polymyxin B caused cells to be elongated rods stained with both FM4-64 and Sytox green. Bar, 2 m. (B) The time course of membrane permeation by BR003-cecropin A and polymyxin B was measured by following the increase in Sytox green fluorescence (ex ⫽ 485 nm; em ⫽ 520 nm). The concentrations of BR003-cecropin A and polymyxin used corresponded to 2⫻ their MICs. Antimicrobial Peptides against A. baumannii by HTS March 2015 Volume 59 Number 3 ACKNOWLEDGMENT We acknowledge NIH grant R01 AI085581. REFERENCES 1. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538 –582. http://dx .doi.org/10.1128/CMR.00058-07. 2. Gales AC, Jones RN, Forward KR, Liñares J, Sader HS, Verhoef J. 2001. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin Infect Dis 32(Suppl 2):S104 –S113. http://dx.doi.org/10.1086/320183. 3. Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254 – 1263. http://dx.doi.org/10.1086/529198. 4. Cisneros JM, Reyes MJ, Pachón J, Becerril B, Caballero FJ, GarcíaGarmendía JL, Ortiz C, Cobacho AR. 1996. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis 22:1026 –1032. http://dx.doi.org/10.1093/clinids/22 .6.1026. 5. Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. 2007. An outbreak of multidrugresistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 44:1577–1584. http://dx.doi.org/10.1086/518170. 6. Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417– 433. http://dx.doi.org/10.1128/MMBR .00016-10. 7. Tan SY-Y, Chua SL, Liu Y, Høiby N, Andersen LP, Givskov M, Song Z, Yang L. 2013. Comparative genomic analysis of rapid evolution of an extreme-drug-resistant Acinetobacter baumannii clone. Genome Biol Evol 5:807– 818. http://dx.doi.org/10.1093/gbe/evt047. 8. Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. http: //dx.doi.org/10.1371/journal.ppat.1003068. 9. Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206 –1215. http://dx.doi.org/10.1093/jac/dkm357. 10. Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG, Antimicrobial Availability Task Force of the Infectious Diseases Society of America. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657– 668. http://dx.doi.org /10.1086/499819. 11. Perron GG, Zasloff M, Bell G. 2006. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci 273:251–256. http://dx.doi.org /10.1098/rspb.2005.3301. 12. Fournier P-E, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie J-M. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2:e7. http://dx.doi.org /10.1371/journal.pgen.0020007. 13. Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, Engelthaler DM, Keim P. 2013. Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in Acinetobacter. PLoS One 8:e54287. http://dx.doi.org/10.1371/journal.pone.0054287. 14. Saugar JM, Rodríguez-Hernández MJ, de la Torre BG, Pachón-Ibañez ME, Fernández-Reyes M, Andreu D, Pachón J, Rivas L. 2006. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50:1251–1256. http://dx.doi.org/10.1128/AAC.50.4.1251-1256.2006. 15. Ewbank JJ, Zugasti O. 2011. C. elegans: model host and tool for antimicrobial drug discovery. Dis Model Mech 4:300 –304. http://dx.doi.org/10 .1242/dmm.006684. 16. Moy TI, Conery AL, Larkins-Ford J, Wu G, Mazitschek R, Casadei G, Lewis K, Carpenter AE, Ausubel FM. 2009. High-throughput screen for Antimicrobial Agents and Chemotherapy aac.asm.org 1735 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital immunomodulatory effects in hosts owing to their ability to bind lipopolysaccharides (LPS) (14, 25, 30). These qualities make AMPs suitable candidates for further evaluation of their antimicrobial and therapeutic potential. From the pilot screen, we identified 15 cecropin and cecropinlike AMPs that prolonged worm survival in the presence of A. baumannii. Cecropin and the cecropin-like peptides are the most abundant linear AMPs in insects (52). One particular cecropin identified in our screen, BR003-cecropin A, synthesized by Aedes aegypti, has a very low in vitro MIC of ⬍5 g/ml and also exhibits a clear zone of inhibition on bacterial lawns in a disc diffusion assays (Fig. 4A and B). BR003-cecropin A consists of 36 amino acid residues with a molecular mass of 3,600 g/mol. We discovered that BR003-cecropin A has antimicrobial activity against several Gram-negative bacteria, including P. aeruginosa, similar to findings reported for cecropin A from Drosophila melanogaster (70). The absence of tryptophan at the N terminus, as well as an increased number of positively charged amino acids in BR003cecropin A compared to other cecropins, may contribute to the low MIC of this cecropin against A. baumannii. Although BR003cecropin A was not effective against the Gram-positive MRSA strain used in this study, it has been shown that cecropin A isolated from Anopheles gambiae, which lacks a tryptophan residue in the N terminus, is more effective in killing yeast and Gram-positive bacteria than other cecropins with tryptophan residues (71). Cecropin A is a linear ␣-helical cationic peptide that is produced by both invertebrates and vertebrates, and like other AMPs, this small peptide is known to form pores in bacterial membranes (14, 28). Indeed, membrane permeabilization was confirmed in this study. In addition, we have observed that cecropins prolonged the survival of worms in our infection assay at a minimum effective concentration of 2 g/ml, a concentration lower than the in vitro MIC, which ranges from 5 g/ml to 20 g/ml. This suggests that BR003-cecropin A may have additional activities in our assay beyond straightforward killing of the pathogen (72, 73). It has previously been shown that AMPs have immunomodulatory activities. The AMPs are evolutionarily conserved components of the innate immune system of eukaryotes. AMPs are produced by a variety of immune cells of hematopoietic and epithelial origin with the ability to modulate pro- and anti-inflammatory effects (74). It has been reported that cecropins exhibit significant selective cytotoxic and antiproliferative properties (75). In conclusion, we developed a robust high-throughput screening method for antimicrobial drug discovery using a C. elegans-A. baumannii liquid killing model. Using this whole-animal model, we were able to test the efficacy of anti-infective small molecules as well as their toxicity to the host in a single screening assay. This assay identified cecropin AMPs with potent antimicrobial activity against A. baumannii, and one particular AMP, BR003-cecropin A from Aedes aegypti, exhibited a very low MIC. We showed that BR003-cecropin A causes membrane permeability, as predicted by the pore-forming ability of AMPs. The identification of specific hit peptides from the pilot screen indicates that the C. elegans-A. baumannii screening assay can lead to the discovery of small molecules with potent antibiotic properties. The methods described here can be adapted to screen for antibiotics effective against many other pathogens and can advance the drug development pipeline for emerging MDR bacteria. Jayamani et al. 17. 18. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 1736 aac.asm.org 38. Kamentsky L, Jones TR, Fraser A, Bray M-A, Logan DJ, Madden KL, Ljosa V, Rueden C, Eliceiri KW, Carpenter AE. 2011. Improved structure, function and compatibility for CellProfiler: modular highthroughput image analysis software. Bioinformatics 27:1179 –1180. http: //dx.doi.org/10.1093/bioinformatics/btr095. 39. Zhang J, Chung T, Oldenburg K. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. http://dx.doi.org/10.1177/108705719900400206. 40. Altincicek B, Knorr E, Vilcinskas A. 2008. Beetle immunity: identification of immune-inducible genes from the model insect Tribolium castaneum. Dev Comp Immunol 32:585–595. http://dx.doi.org/10.1016/j.dci .2007.09.005. 41. Altincicek B, Vilcinskas A. 2009. Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata. Insect Mol Biol 18:119 – 125. http://dx.doi.org/10.1111/j.1365-2583.2008.00856.x. 42. Vilcinskas A. 2011. Anti-infective therapeutics from the Lepidopteran model host Galleria mellonella. Curr Pharm Des 17:1240 –1245. http://dx .doi.org/10.2174/138161211795703799. 43. Vogel H, Altincicek B, Glöckner G, Vilcinskas A. 2011. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 12:308. http://dx.doi.org/10 .1186/1471-2164-12-308. 44. Mukherjee K, Abu Mraheil M, Silva S, Müller D, Cemic F, Hemberger J, Hain T, Vilcinskas A, Chakraborty T. 2011. Anti-Listeria activities of Galleria mellonella hemolymph proteins. Appl Environ Microbiol 77: 4237– 4240. http://dx.doi.org/10.1128/AEM.02435-10. 45. Sowa-Jasiłek A, Zdybicka-Barabas A, Sta˛czek S, Wydrych J, Mak P, Jakubowicz T, Cytryńska M. 2014. Studies on the role of insect hemolymph polypeptides: Galleria mellonella anionic peptide 2 and lysozyme. Peptides 53:194 –201. http://dx.doi.org/10.1016/j.peptides.2014.01.012. 46. Rajamuthiah R, Fuchs BB, Jayamani E, Kim Y, Larkins-Ford J, Conery A, Ausubel FM, Mylonakis E. 2014. Whole animal automated platform for drug discovery against multi-drug resistant Staphylococcus aureus. PLoS One 9:e89189. http://dx.doi.org/10.1371/journal.pone.0089189. 47. Jorgensen JH, Ferraro MJ. 2009. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49:1749 –1755. http://dx.doi.org/10.1086/647952. 48. Moule MG, Monack DM, Schneider DS. 2010. Reciprocal analysis of Francisella novicida infections of a Drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLoS Pathog 6:e1001065. http://dx.doi.org/10 .1371/journal.ppat.1001065. 49. Silvestro L, Weiser JN, Axelsen PH. 2000. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob Agents Chemother 44:602– 607. http://dx.doi.org/10.1128/AAC.44.3.602-607.2000. 50. Saugar JM, Alarcón T, López-Hernández S, López-Brea M, Andreu D, Rivas L. 2002. Activities of polymyxin B and cecropin A-melittin peptide CA(1-8)M(1-18) against a multiresistant strain of Acinetobacter baumannii. Antimicrob Agents Chemother 46:875– 878. http://dx.doi.org/10 .1128/AAC.46.3.875-878.2002. 51. Nonejuie P, Burkart M, Pogliano K, Pogliano J. 2013. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc Natl Acad Sci U S A 110:16169 –16174. http://dx.doi .org/10.1073/pnas.1311066110. 52. Cavallarin L, Andreu D, San Segundo B. 1998. Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant Microbe Interact 11:218 –227. http://dx.doi.org/10.1094/MPMI.1998.11.3.218. 53. Dorsey CW, Beglin MS, Actis LA. 2003. Detection and analysis of iron uptake components expressed by Acinetobacter baumannii clinical isolates. J Clin Microbiol 41:4188 – 4193. http://dx.doi.org/10.1128/JCM.41 .9.4188-4193.2003. 54. Eijkelkamp BA, Hassan KA, Paulsen IT, Brown MH. 2011. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics 12:126. http://dx.doi.org/10.1186/1471-2164-12 -126. 55. Nwugo CC, Gaddy JA, Zimbler DL, Actis LA. 2011. Deciphering the iron response in Acinetobacter baumannii: a proteomics approach. J Proteomics 74:44 –58. http://dx.doi.org/10.1016/j.jprot.2010.07.010. 56. Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. 2004. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 150:3657–3667. http://dx.doi.org /10.1099/mic.0.27371-0. Antimicrobial Agents and Chemotherapy March 2015 Volume 59 Number 3 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital 19. novel antimicrobials using a whole animal infection model. ACS Chem Biol 4:527–533. http://dx.doi.org/10.1021/cb900084v. Tan MW, Mahajan-Miklos S, Ausubel FM. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A 96:715–720. http://dx.doi.org /10.1073/pnas.96.2.715. Garsin DA, Sifri CD, Mylonakis E, Qin X, Singh KV, Murray BE, Calderwood SB, Ausubel FM. 2001. A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A 98:10892– 10897. http://dx.doi.org/10.1073/pnas.191378698. Desalermos A, Tan X, Rajamuthiah R, Arvanitis M, Wang Y, Li D, Kourkoumpetis TK, Fuchs BB, Mylonakis E. 2015. A multi-host approach for the systematic analysis of virulence factors in Cryptococcus neoformans. J Infect Dis 211:298 –305. http://dx.doi.org/10.1093/infdis /jiu441. Rajamuthiah R, Mylonakis E. 2014. Effector triggered immunity: activation of innate immunity in metazoans by bacterial effectors. Virulence 5:697–702. http://dx.doi.org/10.4161/viru.29091. Marsh EK, May RC. 2012. Caenorhabditis elegans, a model organism for investigating immunity. Appl Environ Microbiol 78:2075–2081. http://dx .doi.org/10.1128/AEM.07486-11. Kaletta T, Hengartner MO. 2006. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5:387–398. http://dx .doi.org/10.1038/nrd2031. Mylonakis E, Casadevall A, Ausubel FM. 2007. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog 3:e101. http://dx.doi.org/10.1371/journal .ppat.0030101. Dolla NK, Chen C, Larkins-Ford J, Rajamuthiah R, Jagadeesan S, Conery AL, Ausubel FM, Mylonakis E, Bremner JB, Lewis K, Kelso MJ. 2014. On the mechanism of berberine–INF55 (5-nitro-2-phenylindole) hybrid antibacterials. Aust J Chem 67:1471. http://dx.doi.org/10.1071 /CH14426. Wiesner J, Vilcinskas A. 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440 – 464. http://dx.doi.org/10 .4161/viru.1.5.12983. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389 –395. http://dx.doi.org/10.1038/415389a. Fjell CD, Hiss JA, Hancock REW, Schneider G. 2012. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51. http://dx.doi.org/10.1038/nrd3591. Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238 –250. http://dx.doi.org/10 .1038/nrmicro1098. Yeaman MR. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55. http://dx.doi.org/10.1124/pr.55.1.2. Vilcinskas A, Mukherjee K, Vogel H. 2012. Expansion of the antimicrobial peptide repertoire in the invasive ladybird Harmonia axyridis. Proc R Soc B Biol Sci 280:20122113. http://dx.doi.org/10.1098/rspb.2012.2113. Hilchie AL, Wuerth K, Hancock REW. 2013. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9:761–768. http://dx.doi.org/10.1038/nchembio.1393. Bowdish DME, Davidson DJ, Scott MG, Hancock REW. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob Agents Chemother 49:1727–1732. http://dx.doi.org/10.1128/AAC.49.5 .1727-1732.2005. Beanan MJ, Strome S. 1992. Characterization of a germ-line proliferation mutation in C. elegans. Development 116:755–766. Miyata S, Begun J, Troemel ER, Ausubel FM. 2008. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 178:903–918. http://dx.doi.org/10.1534/genetics.107.083923. Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan M-W, Ausubel FM. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623– 626. http://dx.doi.org/10 .1126/science.1073759. Anastassopoulou CG, Fuchs BB, Mylonakis E. 2011. Caenorhabditis elegans-based model systems for antifungal drug discovery. Curr Pharm Des 17:1225–1233. http://dx.doi.org/10.2174/138161211795703753. Kirienko NV, Kirienko DR, Larkins-Ford J, Wählby C, Ruvkun G, Ausubel FM. 2013. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 13:406 – 416. http://dx.doi.org/10.1016/j.chom.2013.03.003. Antimicrobial Peptides against A. baumannii by HTS March 2015 Volume 59 Number 3 68. 69. 70. 71. 72. 73. 74. 75. 76. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J Antimicrob Chemother 61:568 –576. http://dx.doi.org/10.1093/jac/dkm498. Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Del Prete MS, Łukasiak J, Scalise G. 2003. Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1-7)M(2-9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24:1315–1318. http://dx.doi.org/10.1016/j.peptides.2003.08.003. Fox MA, Thwaite JE, Ulaeto DO, Atkins TP, Atkins HS. 2012. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides 33:197–205. http://dx.doi.org /10.1016/j.peptides.2012.01.013. Hetru C, Troxler L, Hoffmann JA. 2003. Drosophila melanogaster antimicrobial defense. J Infect Dis 187(Suppl 2):S327–S334. http://dx.doi.org /10.1086/374758. Wang Y-P, Lai R. 2010. Insect antimicrobial peptides: structures, properties and gene regulation. Zool Res 31:27–34. http://dx.doi.org/10.3724 /SP.J.1141.2010.01027. Cai Y, Cao X, Aballay A. 2014. Whole-animal chemical screen identifies colistin as a new immunomodulator that targets conserved pathways. mBio 5:e01235-14. http://dx.doi.org/10.1128/mBio.01235-14. Pukkila-Worley R, Feinbaum R, Kirienko NV, Larkins-Ford J, Conery AL, Ausubel FM. 2012. Stimulation of host immune defenses by a small molecule protects C. elegans from bacterial infection. PLoS Genet 8:e1002733. http://dx.doi.org/10.1371/journal.pgen.1002733. Mansour SC, Pena OM, Hancock REW. 2014. Host defense peptides: front-line immunomodulators. Trends Immunol 35:443– 450. http://dx .doi.org/10.1016/j.it.2014.07.004. Suttmann H, Retz M, Paulsen F, Harder J, Zwergel U, Kamradt J, Wullich B, Unteregger G, Stöckle M, Lehmann J. 2008. Antimicrobial peptides of the cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol 8:5. http://dx.doi.org/10.1186/1471-2490 -8-5. Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53: 2605–2609. http://dx.doi.org/10.1128/AAC.01533-08. Antimicrobial Agents and Chemotherapy aac.asm.org 1737 Downloaded from http://aac.asm.org/ on December 30, 2015 by Massachusetts General Hospital 57. Ruzin A, Keeney D, Bradford PA. 2007. AdeABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Antimicrob Chemother 59:1001–1004. http://dx.doi.org/10.1093/jac/dkm058. 58. Rangarajan N, Bakshi S, Weisshaar JC. 2013. Localized permeabilization of E. coli membranes by the antimicrobial peptide cecropin A. Biochemistry 52:6584 – 6594. http://dx.doi.org/10.1021/bi400785j. 59. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. http://dx.doi.org/10.1086/595011. 60. Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079 –1081. http://dx .doi.org/10.1086/533452. 61. Chopra S, Torres-Ortiz M, Hokama L, Madrid P, Tanga M, Mortelmans K, Kodukula K, Galande AK. 2010. Repurposing FDA-approved drugs to combat drug-resistant Acinetobacter baumannii. J Antimicrob Chemother 65:2598 –2601. http://dx.doi.org/10.1093/jac/dkq353. 62. Go ES, Urban C, Burns J, Kreiswirth B, Eisner W, Mariano N, MosinkaSnipas K, Rahal JJ. 1994. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet 344:1329 –1332. http://dx.doi.org/10.1016/S0140-6736(94)90694-7. 63. Karageorgopoulos DE, Falagas ME. 2008. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 8:751–762. http://dx.doi.org/10.1016/S1473-3099(08)70279-2. 64. Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC, Mylonakis E. 2008. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci U S A 105:14585–14590. http: //dx.doi.org/10.1073/pnas.0805048105. 65. Smith MG, Des Etages SG, Snyder M. 2004. Microbial synergy via an ethanol-triggered pathway. Mol Cell Biol 24:3874 –3884. http://dx.doi.org /10.1128/MCB.24.9.3874-3884.2004. 66. Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21:601– 614. http://dx.doi.org/10.1101/gad.1510307. 67. Kawamura-Sato K, Wachino J-I, Kondo T, Ito H, Arakawa Y. 2008.