* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Center for Drug Evaluation and Research
Pharmacokinetics wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Drug interaction wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
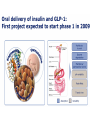
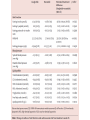
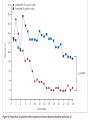
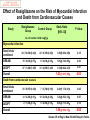
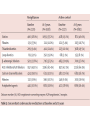
Journal Club John B Buse, Julio Rosenstock, Giorgio Sesti, Wolfgang E Schmidt, Eduard Montanya, Jason H Brett, Marcin Zychma, Lawrence Blonde, for the LEAD-6 Study Group* Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet online June 8, 2009 Philip D Home, Stuart J Pocock, Henning Beck-Nielsen, Paula S Curtis, Ramon Gomis, Markolf Hanefeld, Nigel P Jones, Michel Komajda, John J V McMurray, for the RECORD Study Team* Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial Lancet online June 5, 2009 2009年6月18日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi Division of Endocrinology, University of North Carolina School of Medicine, Chapel Hill, NC, USA (Prof J B Buse MD); Dallas Diabetes and Endocrine Center at Medical City, Dallas, TX, USA (J Rosenstock MD); Department of Experimental and Clinical Medicine, Magna Graecia University of Catanzaro, Catanzaro, Italy (Prof G Sesti MD); Department of Medicine I, St Josef-Hospital, Ruhr-University Medical Faculty, Bochum, Germany (Prof W E Schmidt MD); Hospital Universitari Bellivtge-IDIBELL, University of Barcelona, CIBER de Diabetes y Enfermedades Metabolicas Asociadas (CIBERDEM), Barcelona, Spain (Prof E Montanya MD); Novo Nordisk, Princeton, NJ, USA (J H Brett MD); Novo Nordisk, Bagsvaerd, Denmark (M Zychma MD); and Ochsner Diabetes Clinical Research Unit, Department of Endocrinology, Ochsner Medical Center, New Orleans, LA, USA (L Blonde MD) www.thelancet.com Published online June 8, 2009 LEAD: Liraglutide Effect and Action in Diabetes. All studies 26 weeks’ duration (LEAD 3=52 weeks); all RCT; all with double dummy except LEAD 5 vs. glargine. Studies NN2211-1436, 1572, -1573 and -1697 presented as Marre et al. Diabetes 2008;57(Suppl. 1):A4 (LEAD 1); Nauck et al. Diabetes 2008;57(Suppl. 1):A150 (LEAD 2); Garber et al, The Lancet, accepted for publication (LEAD 3); Russell-Jones et al. Diabetes 2008;57(Suppl. 1):A159 (LEAD 5). Background Unlike most antihyperglycaemic drugs, glucagon-like peptide-1 (GLP-1) receptor agonists have a glucosedependent action and promote weight loss. We compared the efficacy and safety of liraglutide, a human GLP-1 analogue, with exenatide, an exendinbased GLP-1 receptor agonist. The trial is registered with ClinicalTrials.gov, number NCT00518882. Methods Adults with inadequately controlled type 2 diabetes on maximally tolerated doses of metformin, sulphonylurea, or both, were stratified by previous oral antidiabetic therapy and randomly assigned to receive additional liraglutide 1・8 mg once a day (n=233) or exenatide 10 μg twice a day (n=231) in a 26-week open-label, parallelgroup, multinational (15 countries) study. The primary outcome was change in glycosylated haemoglobin (HbA1c). Efficacy analyses were by intention to treat. Figure 1: Trial profi le Of the adverse events leading to withdrawal, nausea was the most common (14 patients in the liraglutide group and 16 in the exenatide group). Participants were exposed to treatment if they had received at least one dose of study medication. Figure 2: Efficacy of treatment with liraglutide 1・8 mg once a day or exenatide 10 μg twice a day (A) Glycosylated haemoglobin (HbA1c) values from baseline to week 26. (B) Change in HbA1c values from baseline to week 26. (C) Percentage of patients achieving HbA1c target values. (D) Fasting plasma glucose (FPG) concentrations from baseline to week 26. (E) 7-point self-measured plasma glucose profi les. (F) Bodyweight from baseline to week 26. Data are mean (1・96 SE) unless stated otherwise, with last observation carried forward (except for panel E, observed case). Figure 2: Efficacy of treatment with liraglutide 1・8 mg once a day or exenatide 10 μg twice a day (A) Glycosylated haemoglobin (HbA1c) values from baseline to week 26. (B) Change in HbA1c values from baseline to week 26. (C) Percentage of patients achieving HbA1c target values. (D) Fasting plasma glucose (FPG) concentrations from baseline to week 26. (E) 7-point self-measured plasma glucose profi les. (F) Bodyweight from baseline to week 26. Data are mean (1・96 SE) unless stated otherwise, with last observation carried forward (except for panel E, observed case). Figure 2: Efficacy of treatment with liraglutide 1・8 mg once a day or exenatide 10 μg twice a day (A) Glycosylated haemoglobin (HbA1c) values from baseline to week 26. (B) Change in HbA1c values from baseline to week 26. (C) Percentage of patients achieving HbA1c target values. (D) Fasting plasma glucose (FPG) concentrations from baseline to week 26. (E) 7-point self-measured plasma glucose profi les. (F) Bodyweight from baseline to week 26. Data are mean (1・96 SE) unless stated otherwise, with last observation carried forward (except for panel E, observed case). Data are number (%) of participants. Liraglutide group: adenocarcinoma of the pancreas, adenocarcinoma of the lung, thyroid neoplasm, coronary artery stenosis, supraventricular tachycardia, cerebellar infarction, sciatica, diplopia, cholelithiasis, pneumonia, postmenopausal vaginal bleeding, and asthma. Exenatide group: acute myocardial infarction, coronary artery disease, cerebrovascular accident, cataract, arthralgia, and hypoglycaemia. Table 3: Treatment-emergent adverse events Figure 4: Number of minor hypoglycaemic episodes at 2-h intervals Liraglutide 1・8 mg once a day or exenatide 10 μg twice a day. Results Mean baseline HbA1c for the study population was 8・2%. Liraglutide reduced mean HbA1c significantly more than did exenatide (–1・12% [SE 0・08] vs –0・79% [0・08]; estimated treatment difference –0・33; 95% CI –0・ 47 to –0・18; p<0・0001) and more patients achieved a HbA1c value of less than 7% (54% vs 43%, respectively; odds ratio 2・02; 95% CI 1・31 to 3・11; p=0・0015). Liraglutide reduced mean fasting plasma glucose more than did exenatide (–1・61 mmol/L [SE 0・20] vs –0・60 mmol/L [0・20]; estimated treatment difference –1・01 mmol/L; 95% CI –1・37 to –0・65; p<0・0001) but postprandial glucose control was less effective after breakfast and dinner. Both drugs promoted similar weight losses (liraglutide –3・24 kg vs exenatide –2・87 kg). Both drugs were well tolerated, but nausea was less persistent (estimated treatment rate ratio 0・448, p<0・0001) and minor hypoglycaemia less frequent with liraglutide than with exenatide (1・93 vs 2・60 events per patient per year; rate ratio 0・55; 95% CI 0・34 to 0・88; p=0・0131; 25・5% vs 33・6% had minor hypoglycaemia). Two patients taking both exenatide and a sulphonylurea had a major hypoglycaemic episode. Conclusion Liraglutide once a day provided significantly greater improvements in glycaemic control than did exenatide twice a day, and was generally better tolerated. The results suggest that liraglutide might be a treatment option for type 2 diabetes, especially when weight loss and risk of hypoglycaemia are major considerations. Funding Novo Nordisk A/S. In 2007 チアゾリジン系薬剤 Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes Study Rosiglitazone Group Control Group Odds Ratio (95% Cl) P Value no. of events / total no.(%) Myocardial infarction Small trials combined 44 / 10,280(0.43) 22 / 6,105(0.36) 1.45(0.88-2.39) 0.15 DREAM 15 / 2,635(0.57) 9 / 2,634(0.34) 1.65(0.74-3.68) 0.22 ADOPT 27 / 1,456(1.85) 41 / 2,895(1.44) 1.33(0.80-2.21) 0.27 1.43(1.03-1.98) 0.03 Overall Death from cardiovascular causes Small trials combined 25 / 6,557(0.38) 7 / 3,700(0.19) 2.40(1.17-4.91) 0.02 DREAM 12 / 2,365(0.51) 10 / 2,634(0.38) 1.20(0.52-2.78) 0.67 ADOPT 2 / 1,456(0.14) 5 / 2,854(0.18) 0.80(0.17-3.86) 0.78 1.64(0.98-2.74) 0.06 Overall Nissen SE.:N Engl J Med.356.2007.May 21.Online Assessment of the cardiovascular risks and health benefits of rosiglitazone David J. Graham, MD, MPH Office of Surveillance and Epidemiology Food and Drug Administration July 30, 2007 Center for Drug Evaluation and Research Joint Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee July 30, 2007 Does CV risk with RSG differ from that with PIO? • Yes • From DREAM, relatively low-risk population: RSG increased risk by ~40% c/w PBO • From PROactive, high risk population: PIO decreased risk by ~15% c/w PBO • From RSG meta-analysis: RSG increased risk of serious IHD by ~40% c/w all comparators & by ~70% c/w PBO • From PIO meta-analysis: PIO decreased risk by ~25% c/w all comparators • From head-to-head GLAI: RSG increased risk 3.5-fold c/w PIO Center for Drug Evaluation and Research Joint Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee July 30, 2007 Center for Drug Evaluation and Research Joint Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee July 30, 2007 アクトスのイベントに及ぼす影響(メタ解析) (%) 10 累 積 イ ベ ン ト 発 症 率 総死亡、心筋梗塞、脳卒中 8 対照群 ピオグリタゾン群 6 4 ハザード比 95%信頼区間 2 ピオグリタゾン vs 対照群 0.82 0.72,0.94 p値 0.005 0 0 症例数 7,836 対照群 アクトス群 8,554 20 40 60 80 100 120 6,470 6,556 5,509 5,370 4,133 4,026 3,735 3,679 3,534 3,505 2,826 2,810 140 (週) 2,143 2,146 19の臨床試験から16,390例を対象にピオグリタゾン群と対照群(プラセボ、SU薬、BG薬、インスリン)における、 イベントの発症率をメタ解析した。 Lincoff A.M. et al.:JAMA,298,1180,2007. 背景別にみたアクトスのイベントに及ぼす影響(メタ解析) ー総死亡、心筋梗塞、脳卒中ー 試験数 ピオグリタゾン群 PROactive試験 その他の試験 1 対照群 0.05 0.1 1.0 5.0 301/2,605(11.6) 358/2,633(13.6) 0.84(0.72-0.98) 18 74/5,949(1.2) 92/5,203(1.8) 3ヵ月- 6ヵ月 5 3/1,131(0.3) 5/742(0.7) 6ヵ月-12ヵ月 4 18/1,136(1.6) 12ヵ月-24ヵ月 8 37/2,631(1.4) 24ヵ月- 2 試験期間別解析 0.75(0.55-1.02) 0.40(0.10-1.68) 0.73(0.38-1.40) 0.80(0.52-1.23) 47/2,630(1.8) 0.85(0.73-0.98) 18/785(2.3) 317/3,656(8.7) 380/3,679(10.3) 0.09(0.01-0.84) 対照薬別解析 プラセボ 3 プラセボ+他剤 6 メトホルミン 2 SU薬 7 ロシグリタゾン 1 全体 ハザード比(95%CI) 対照群 が優れる ピオグリタゾン群 が優れる 19 1/606(0.2) 4/259(1.5) 2/369(0.5) 5/366(1.4) 0.83(0.71-0.97) 305/3,777(8.1) 364/3,425(10.6) 0.61(0.31-1.22) 13/916(1.4) 22/917(2.4) 1.02(0.70-1.48) 0.39(0.08-2.00) 54/2,886(1.9) 55/2,869(1.9) 0.82(0.72-0.94) 375/8,554(4.4) 450/7,836(5.7) 19の臨床試験から16,390例を対象にピオグリタゾン群と対照群(プラセボ、SU薬、BG薬、インスリン)における、 イベントの発症率をメタ解析した。( )内は% Lincoff A.M. et al.:JAMA,298,1180,2007. Adv. Committee 23 members voted Q1.Rosiglitazone increases cardiac risk in patients with type 2 diabetes? ○For 20 ×Against 3 Q2.Rosiglitazone should remain available to physicians and patients? ○For 22 ×Against 1 (weak) ・ label warnings ・ extensive educational efforts The 5½-year study, which was conducted at 338 centers in 23 countries in Europe, Australia, and New Zealand Newcastle Diabetes Centre and Newcastle University, Newcastle upon Tyne, UK (Prof P D Home DM); Medical Statistics Unit, London School of Hygiene and Tropical Medicine, London, UK (Prof S J Pocock PhD); Department of Endocrinology and Metabolism, Odense, Denmark (Prof H Beck-Nielsen DMSC); GlaxoSmithKline Research and Development, Greenford, UK (P S Curtis PhD); Hospital Clinic, University of Barcelona, Barcelona, Spain (Prof R Gomis MD); Zentrum fur Klinische Studien Forschungsbereich Endokrinologie und Stoff wechsel, Dresden, Germany (Prof M Hanefeld MD); GlaxoSmithKline Research and Development, Harlow, UK (N P Jones MA); Universite Pierre et Marie Curie Paris 6; Hopital Pitie-Salpetriere, Departement de Cardiologie, Paris, France (Prof M Komajda MD); and British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK (Prof J J V McMurray MD) www.thelancet.com Published online June 5, 2009 BACKGROUND Rosiglitazone is an insulin sensitiser used in combination with metformin, a sulfonylurea, or both, for lowering blood glucose in people with type 2 diabetes. We assessed cardiovascular outcomes after addition of rosiglitazone to either metformin or sulfonylurea compared with the combination of the two over 5–7 years of follow-up. We also assessed comparative safety. METHODS In a multicentre, open-label trial, 4447 patients with type 2 diabetes on metformin or sulfonylurea monotherapy with mean haemoglobin A1c (HbA1c) of 7・9% were randomly assigned to addition of rosiglitazone (n=2220) or to a combination of metformin and sulfonylurea (active control group, n=2227). The primary endpoint was cardiovascular hospitalisation or cardiovascular death, with a hazard ratio (HR) non-inferiority margin of 1・20. Analysis was by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00379769. Figure 1: Trial profile Table 1: Baseline characteristics of the people with diabetes studied, divided by background treatment stratum and randomised therapy group Data are number (%) or mean (SD). HbA1c=haemoglobin A1c. *Microalbuminuria is defined as albumin to creatinine ratio >2・5 mg/mmol (men) or >3・5 mg/mmol (women). Figure 2: Kaplan-Meier plots of time to the primary endpoint (cardiovascular death or cardiovascular hospitalisation) HR=hazard ratio. Figure 3: Kaplan-Meier plots for components of the primary endpoint (A) All-cause death. (B) CV death. (C) Myocardial infarction. (D) Stroke. (E) CV death, myocardial infarction, and stroke. (F) Heart failure. CV=cardiovascular. HR=hazard ratio. Figure 3: Kaplan-Meier plots for components of the primary endpoint (A) All-cause death. (B) CV death. (C) Myocardial infarction. (D) Stroke. (E) CV death, myocardial infarction, and stroke. (F) Heart failure. CV=cardiovascular. HR=hazard ratio. Figure 3: Kaplan-Meier plots for components of the primary endpoint (A) All-cause death. (B) CV death. (C) Myocardial infarction. (D) Stroke. (E) CV death, myocardial infarction, and stroke. (F) Heart failure. CV=cardiovascular. HR=hazard ratio. Table 5: Patients with events (numbers of events) for various cardiovascular hospitalisations or deaths Data are all events not just first events, and so may add up to higher numbers than those given in table 4. *Fatal events of unknown cause were regarded as being of cardiovascular origin, unless evidence existed to adjudicate them otherwise. Figure 4: Hazard ratios for the primary endpoint on the basis of prespecified subgroups according to particular baseline characteristics of interest ACE=angiotensin-converting enzyme. BMI=body-mass index. Table 6: Patients with serious adverse events Data are number of patients (%). Data are for serious adverse events reported for more than 20 people or those predefined as being of particular interest in the context of thiazolidinedione therapy. *For prostate cancer, data are for men only, and for breast cancer data are for women only. †For non-serious adverse events and details, see table 7 and text. ‡For non-serious adverse events, see text. RESULTS Rosiglitazone is an insulin sensitiser used in combination with metformin, a sulfonylurea, or both, for lowering blood glucose in people with type 2 diabetes. We assessed cardiovascular outcomes after addition of rosiglitazone to either metformin or sulfonylurea compared with the combination of the two over 5–7 years of follow-up. We also assessed comparative safety. CONCLUSION Addition of rosiglitazone to glucose-lowering therapy in people with type 2 diabetes is confirmed to increase the risk of heart failure and of some fractures, mainly in women. Although the data are inconclusive about any possible effect on myocardial infarction, rosiglitazone does not increase the risk of overall cardiovascular morbidity or mortality compared with standard glucose-lowering drugs. Funding GlaxoSmithKline plc, UK. The effects of pioglitazone and rosiglitazone on cardiovascular outcomes will be assessed in a direct comparison in the thiazolidinedione intervention with vitamin D evaluation (TIDE) study (ClinicalTrials.gov NCT00879970).