* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1315_Ansell_EB4E2
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
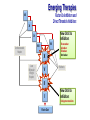
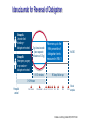
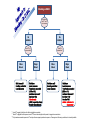
New Reversal Agents for the New Oral Anticoagulants Jack Ansell, MD Hofstra North Shore/LIJ School of Medicine Nov 4, 2016 Disclosures CONSULTANT: Bristol Myers Squibb, Pfizer, Boehringer Ingelheim, Daiichi, Janssen SPEAKER: BMS/Pfizer; Boehringer Ingelheim; Daiichi SCIENTIFIC ADVISORY BOARDS: Instrumentation Laboratories, Perosphere EQUITY: Perosphere Emerging Therapies Factor Xa Inhibitors and Direct Thrombin Inhibitors XII XI New Oral Xa Inhibitors IX VII VIII Unfractionated Heparin X Low Molecular Weight Heparin V Rivaroxaban Apixaban Edoxaban Betrixaban Warfarin II I Fibrin Clot New Oral IIa Inhibitors Dabigatran etexilate PK and other attributes of warfarin vs DOACs Features Warfarin DOACs Onset Slow Rapid Dosing Variable Fixed Food effect Drug interactions (some variability) Yes No Many Few (but can be managed) (difficult to manage) Monitoring Yes Half-life Long Antidote Yes No (but some questions) Short (with normal renal fx) Yes (eventually) What might reversal agents do? • Improve outcomes from major or life-threatening bleeding. • Allow for emergent surgery when drug is onboard. • Reassure physicians leading to greater use of AC • Reassure patients leading to peace of mind (? improved adherence). • Effectively treat overdose. • Improve management of brief interruptions of therapy for invasive procedure. Reversal Agents in Development Company Agent Target Phase BoehringerIngelheim Idarucizumab: Fully humanized Fab Dabigatran only III Approved Portola Pharmaceuticals, Inc. Andexanet alfa: Recombinant, modified human Factor Xa Factor Xa Inhibitors (Riva; Apix; Edox & LMWH) III All NOACs (Dabi; Riva; Apix; Edox) UFH, LMWH, fondaparinux II Ciraparantag: Perosphere Inc. (PER977; Aripazine) Di-arginine piperazine Idarucizumab: Dabigatran Reversal Agent Under Development • Humanized antibody fragment specific for dabigatran (Boehringer Ingelheim Pharma GmbH & Co, Biberach, Germany) – Affinity for dabigatran 350 x the affinity for thrombin – In vitro human plasma studies • Fab had no effect on thrombin generation or coagulation studies – Dose-ranging animal studies • IV administration produced a rapid, dose-dependent decrease in blood loss that was maintained for 6 hrs after the largest dose. • Ex vivo coagulation assays (aPTT, TT, ECT) were reversed. – Phase I human studies completed 1. Van Ryn J, et al. Circulation. 2012;126: A9928. 2. Schiele F, et al. Blood 2013; Idarucizumab for Reversal of Dabigatran Pollack et al. N Engl J Med 2015;373:511-520 REVERSE-AD: Idarucizumab for Reversal of Dabigatran Multicenter, ongoing, open-label, single-arm phase III study Group A: Uncontrolled bleeding + dabigatran-treated Group B: Emergency surgery or procedure + dabigatran-treated 5 g idarucizumab (two separate infusions of 2.5 g) 0–15 minutes Reverses up to the 99th percentile of dabigatran levels measured in RE-LY N=300 90 days follow-up 0–24 hours Hospital arrival Pre-1st vial Pre-2nd vial ~20 min 1 h 2 h 4 h 12 h 24 h 30 d 90 d Blood samples Pollack et al. N Engl J Med 2015;373:511-520 Idarucizumab for Reversal of Dabigatran Select patient characteristics Group A Group B Pollack et al. N Engl J Med 2015;373:511-520 Idarucizumab produced sustained reversal of dabigatran Bleeding Patients Surgical Patients Pollack et al. N Engl J Med 2015;373:511-520 Secondaryproduced Endpoints: Clinical Outcomes Idarucizumab “potentially” good outcomes Group A 51 Patients Group B 39 Patients Assessable in 38 patients Surgery performed in 36 patients Median local investigatordetermined time to bleeding cessation 11.4 hours* Intraoperative hemostasis: • 33 normal • 2 mildly abnormal • 1 moderately abnormal *Assessment of bleeding cessation may be difficult wotj internal bleeding into confined space such as intramuscular or intracranial bleeding. Pollack et al. N Engl J Med 2015;373:511-520 RE-VERSE AD: Updated Interim Analysis(ACC 4/16) 123 Patients • Group A 66 Patients • Median time to bleeding cessation was 9.8 hours (investigatorreported among assessed patients) • Group B 57 Patients • Mean time to surgery was 1.7 hours following administration of idarucizumab. • Normal hemostasis) during surgery reported in 92% percent of patients (48/52). • Thrombotic events occurred in five patients between 2-24 days after idarucizumab administration. None of these patients were receiving antithrombotic therapy at the time of their event. • 26 deaths, which appear to have been related to reason for emergency admission to the hospital and/or to co-morbidities 12 Pollack et al. ACC abstract April 2016 Praxbind prescribing information Andexanet: Factor Xa Inhibitor Reversal Agent Under Development • Andexanet (PRT064445; aka PRT4445; Portola, South San Francisco) – – – – Recombinant protein (r-Antidote) (Chinese hamster ovary cells) Binds to both direct- and indirect-acting Factor Xa inhibitors Catalytically inactive Ex vivo studies • Reverses Factor Xa inhibition in dose-dependent manner • Corrects prolongation of clotting times – Restores hemostasis in animal models of blood loss associated with Factor Xa inhibitors – Phase I study • 32 healthy volunteers, “Generally safe and well-tolerated” • Reversed anticoagulation within 5 min and was sustained for 3 hrs – Phase III study ongoing with apixaban and rivaroxaban 1.Lu G, et al. Nat Med. 2013; 2. Portola Pharmaceuticals. Portola initiates phase 2 study of PRT4445, universal antidote for factor Xa inhibitor anticoagulants. December 10, 2012. 3. Clinicaltrials.gov database. http://www.clinicaltrials.gov/ct2/show/NCT01758432?term=PRT064445&rank=1 Siegal et al. N Eng J Med. pub Nov 11, 2015 online; 10.1056/NEJMoa1510991 Andexanet reversal of Xa Inhibitors in healthy volunteers Time course of anti-factor Xa activity before and after administration of a bolus or bolus + infusion of andexanet alfa in healthy elderly volunteers treated with apixaban or rivaroxaban Siegal DM et al. N Engl J Med 2015; 373:2413-2424 NEJM published August 30, 2016’ DOI: 10.1056/NEJMoa1607887 Anti–Factor Xa Activity and Percent Change from Baseline in Patients Andexanet effect on anti-Xa assay Receiving Rivaroxaban and Apixaban (Efficacy Population). in patients with bleeding Connolly SJ et al. N Engl J Med 2016. DOI: 10.1056/NEJMoa1607887 Subgroup Hemostatic Efficacy. Andexanet effect onAnalysis majorof bleeding Andexanet achieved hemostatic efficacy at 12 hrs in 79% of patients (37/47) Connolly SJ et al. N Engl J Med 2016. DOI: 10.1056/NEJMoa1607887 Ciraparantag (PER977): Factor Xa & Direct Thrombin Inhibitor Reversal Agent Under Development Synthetic, small molecule that non-covalently binds oral direct Xa and IIa inhibitors, unfractionated heparin, LMWH, and fondaparinux Reduces blood loss and bleeding time, reversing the anticoagulant activity of dabigatran, rivaroxaban, apixaban and edoxaban in rat tail transection and liver laceration assays Does not bind to a range of cardiovascular, antiepileptic and anesthetic drugs Stable as a ready-to-use solution for 2 years, refrigerated Laulicht B et al. Circulation. 2012;126:A11395. Laulicht B et al. Small molecule antidote for anticoagulants. Available at: www.perosphere.com/pdf/PER977_AHA-presentation.pdf. Accessed 1/3/14. Ciraparantag (PER977) reverses edoxaban In a phase I/II study PER977 restored normal clotting after single IV doses of 25, 100 and 300 mg vs placebo 3 hours following a single oral dose of edoxaban 60 mg Change from baseline whole blood clotting time (WBCT) 50% PER977 Administered 60 mg edoxaban Placebo reversal 25 mg PER977 aripazine reversal 100 mg PER977 aripazine reversal PER977 reversal 300 mg aripazine 40% 30% * p < 0.05 vs placebo 20% * * *** * * * * * 10% * * 0% 0 -10% 3 6 9 12 15 18 21 24 27 Hours post edoxaban administration Ansell et al. N Engl J Med 2014;371:2141-2142 Ciraparantag (PER977) reverses steady-state edoxaban, allows for re-anticoagulation and re-reversal Reversal of steady-state edoxaban dosing Re-anticoagulation with edoxaban and re-reversal with PER 977 Ciraparantag (PER977) reverses enoxaparin 45% 1.5mg/kg s.c. enoxaparin Change from baseline WBCT ciraparantag 40% Saline placebo 35% 100mg i.v. ciraparantag 200mg i.v. ciraparantag 30% 300mg i.v. ciraparantag 25% 20% 15% 10% 5% 0% 0 4 8 12 16 20 24 28 -5% -10% Hours post enoxaparin administration Ansell et al. Thromb Res 2016 (in press) Bleeding on DOAC If drug unknown check TT and PT* Check creatinine IIa Inhibitor Xa Inhibitor Check PTT or TT to estimate effect** Minor Bleed Hold dose until bleeding controlled Local measures Check PT to estimate effect*** Major Bleed Hold dose Local measures Transfusion as needed (RBC or Plt) Activated charcoal if last dose < 2hr Idarucizumab (aPCC a possible altern) Consider hemodialysis Minor Bleed Hold dose until bleeding controlled Local measures Major Bleed Hold dose Local measures Transfusion as needed (RBC or Plt) Activated charcoal if last dose < 2hr Consider PCC Future: andexanet or ciraparantag * Normal TT suggests Xa inhibitor or IIa inhibitor at negligible concentration; ** Normal TT= negligble IIa inhibitor present; normal PTT does not exlcude significant IIa present, but suggests low concentration; ***Only rivaroxaban somewhat responsive to PT and only with some reagents; apixaban not responsive. Chromogenic anti-Xa assay is quantitative, but not readily available. Thank you!