* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chromatography
Survey
Document related concepts
Transcript
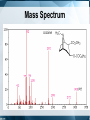
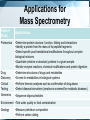
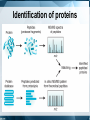
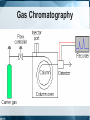
Chromatography: HPLC, HPSEC, MS, LC-MS/MS, GC Isariya Techatanawat, PhD Director of Bioequivalence Study Group, Research and Development Institute, The Government Pharmaceutical Organization Chromatography • Chromatography is a technique for separating and/or identifying the components in a mixture. • Components in a mixture have different tendencies to adsorb onto a surface or dissolve in a solvent. • All chromatographic methods require one static part (stationary phase) and one moving part (mobile phase). Type of Chromatography 1. 2. 3. 4. Adsorption Chromatography Partition Chromatography Ion Exchange Chromatography Size Exclusion Chromatography 1. Adsorption Chromatography • Solid stationary phase • Liquid or gaseous mobile phase • Each solute has its own equilibrium between adsorption onto the surface of the solid and solubility in the solvent, the least soluble or best adsorbed ones travel more slowly. • The result is a separation into bands containing different solutes. 1. Adsorption Chromatography 2. Partition Chromatography • Stationary phase is a non-volatile liquid. • Mixture to be separated is carried by gas or liquid as mobile phase. • Solutes distribute themselves between the moving and the stationary phases, with more soluble component in mobile phase reaching the end of chromatography column first. 2. Partition Chromatography 3. Ion-Exchange Chromatography (IEC) • Ion-exchange chromatography based upon electrical charge. • Likes may repel, while opposites are attracted to each other. • Stationary phases are characterized by nature and strength of acidic or basic functions on their surfaces and the types of ions that they attract and retain. 3. Ion-Exchange Chromatography (IEC) • Opposite charge are electrostatically bound to the surface. • When the mobile phase is eluted through resin, electrostatically bound ions are released as other ions are bonded preferentially Ion-Exchange Chromatography (IEC) • Cation exchange is used to retain and separate positively charged ions on a negative surface. • Anion exchange is used to retain and separate negatively charged ions on a positive surface. 3. Ion-Exchange Chromatography (IEC) 3. Ion-Exchange Chromatography (IEC) • Adsorption based on binding of opposite charges. • Different components (virus, proteins, DNA) have different charges, which means strength of binding varies from component to component. 4. Size Exclusion Chromatography • Mixture passes as a gas or a liquid through a porous gel. • Pore size is designed to allow the large solute particles to pass through uninhibited. • Small particles permeate gel and are slowed down. The smaller the particles, the longer it takes for them to get through column. • Separation is according to particle size. 4. Size Exclusion Chromatography HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) Principles of Liquid Chromatography HPLC • HPLC is improved form of column chromatography. • Smaller particle size for column packing material • Provide greater surface area for interactions between stationary phase and the molecules. • Allow better separation of mixture component HPLC • Instead of solvent being allowed to drip through a column under gravity, it is forced through under high pressures. • To separate, identify, quantitate compounds. • Compounds in trace concentrations as low as parts per trillion [ppt] may also easily be identified. HPLC • Normal Phase HPLC • Reversed Phase HPLC Normal Phase HPLC • Stationary phase is polar (eg silica particles). • Mobile phase is non-polar solvent (eg. Hexane). Normal Phase HPLC • Stationary phase is polar and retains polar yellow dye most strongly. • Non-polar blue dye is won in the retention competition by the mobile phase and elutes quickly. Reversed Phase HPLC • Stationary phase is non-polar. Silica is modified to make it non-polar by attaching long hydrocarbon chains to its surface (eg. C8 or C18 carbon atoms). • Mobile phase is polar solvent (eg. mixture of water and methanol). Reversed Phase HPLC • The most strongly retained compound is non-polar blue dye, as its attraction to nonpolar stationary phase. • Polar yellow dye is won in competition by the polar, aqueous mobile phase, moves the fastest, and elutes earliest. HPLC HPLC Separation What is Chromatogram? HPLC: Qualitative Analysis HPLC: Quantitative Analysis HPLC Chromatogram Isocratic LC System Gradient LC System Gradient LC System Preparative Chromatography High Performance Size Exclusion Chromatography (HPSEC) Size Exclusion Chromatography (SEC) • Smaller molecules penetrate more of the pores on their passage through the bed. • Larger molecules may penetrate pores above a certain size so they spend less time in the bed. • The larger molecules elute first, while the smaller molecules travel slower [because they move into and out of more of the pores] and elute later. Size Exclusion Chromatography (SEC) Size Exclusion Chromatography (SEC) • Biomolecules could be separated based on their size, by passing, or filtering, them through a controlled-porosity packing. • Stationary phases are synthesized with a pore-size distribution. • Mobile phases are good solvents for analytes and may prevent any interactions [based on polarity or charge] between analytes and stationary phase surface. Size Exclusion Chromatography (SEC) • Large components such as virus typically elute just after void volume. • Contaminating proteins and nucleic acids elute at volume greater than void volume. SEC: Exclusion Limit • SEC resin are often characterized by their exclusion limit. • Exclusion limit is the molecular weight of the smallest molecule which cannot enter the pores of the matrix. • All molecules bigger than the exclusion limit elute in the void volume. Typical steps in SEC • Equilibration – prepare column for binding target biomolecule/virus • Load – apply biomolecule/virus to column • Elution – collect purified biomolecule/virus from column • Cleansing/Sanitization – ensure no viable microorganisms present • Storage – fill column with solution that minimizes biological growth and maintains integrity of resin Separation by SEC HPSEC HPSEC HPSEC • Chromatogram shows how much material exited the column at any one time, with the higher molecular weight, larger polymer coils eluting first, followed by successively lower molecular weight (and therefore smaller) chains emerging later. • The primary separation is according to elution volume. HPSEC • Chromatogram is compared to calibration that shows the elution behavior of a series of polymers for which the molecular weight is known. • Molecular weight distribution of the sample is calculated. Calibration graph used to determine polymer MW from its retention time Average molecular weights of polymer nearly symmetrical Process flow for the preparation of clinical flu vaccine Expansion of Vero cells Infection Harvest and Clearance Benzonase TFF AIEX SEC Purified Vaccine Bulk Mass Spectrometry (MS) Mass Spectrometry • Measures mass-to-charge ratio of ions to identify and quantify molecules. Mass spectrometer 1. Ion source: Sample molecules are ionised. 2. Mass analyzer: Sample molecules are separated according to their mass/charge ratio (m/z). 3. Ion detector: Separated ions are detected and sent to data system. m/z ratios and their relative abundance is presented as m/z spectrum . Ionisation methods • Electrospray Ionisation (ESI) • Matrix Assisted Laser Desorption Ionisation (MALDI) • Atmospheric Pressure Chemical Ionisation (APCI) • Chemical Ionisation (CI) • Electron Impact (EI) • Fast Atom Bombardment (FAB) • Field Desorption / Field Ionisation (FD/FI) • Thermospray Ionisation (TSP) Mass Analyser • To separate ions formed in ionisation source according to their mass-to-charge (m/z) ratios. • Mass analysers: quadrupoles, time-offlight (TOF) analysers, magnetic sectors , and both Fourier transform and quadrupole ion traps. Ion Detector • To monitor ion current and amplify it. • Signal is transmitted to data system where it is recorded as mass spectra . • m/z values of ions are plotted against their intensities to show number of components in sample, molecular mass of each component, and relative abundance of various components in sample. Mass Spectrometer Mass Spectrum Mass Spectrum Mass Spectrometry • m/z spectrum shows dominant ions at m/z 556.1, which are consistent with the expected protonated molecular ions, (M+H+). • Measured molecular weight is 555.1 Da. Mass spectrum of sulfamethazine acquired without collision-induced dissociation exhibits little fragmentation Mass spectrum of sulfamethazine acquired with collision-induced dissociation exhibits more fragmentation and more structural information Mass Spectrum Mass Spectrometry • Samples (M) with molecular weights greater than 1200 Da give rise to multiply charged molecular-related ions such as (M+nH)n+ in positive ionisation mode and (M-nH)n- in negative ionisation mode. Mass Spectrometry • Proteins have many suitable sites for protonation as all of the backbone amide nitrogen atoms could be protonated theoretically, as well as certain amino acid side chains such as lysine and arginine which contain primary amine functionalities. Applications for Mass Spectrometry Field of Study Applications Proteomics •Determine protein structure, function, folding and interactions •Identify a protein from the mass of its peptide fragments •Detect specific post-translational modifications throughout complex biological mixtures •Quantitate (relative or absolute) proteins in a given sample •Monitor enzyme reactions, chemical modifications and protein digestion Drug Discovery •Determine structures of drugs and metabolites •Screen for metabolites in biological systems Clinical Testing •Perform forensic analyses such as confirmation of drug abuse •Detect disease biomarkers (newborns screened for metabolic diseases) Genomics •Sequence oligonucleotides Environment •Test water quality or food contamination Geology •Measure petroleum composition •Perform carbon dating Tandem (MS/MS) mass spectrometers • More than one analyser. – quadrupole-quadrupole – magnetic sector-quadrupole – quadrupole-time-of-flight geometries. LC-MS LC-MS/MS LC-MS vs LC-MS/MS LC-MS/MS Full scan mass spectrum of ginsenoside Rb1 showing primarily sodium adduct ions Full scan product ion (MS/MS) spectrum from the sodium adduct at m/z 1131.7 Subsequent full scan product ion spectrum (MS3) from the ion at m/z 789.7 LC-MS/MS Identification of proteins Identification of proteins Biochemical Applications • Rapid protein identification using capillary LC/MS/MS and database searching Molecular Weight Determination Determining molecular weight of green fluorescent protein • 27,000 Dalton with 238 amino acids. Rapid protein identification using capillary LC/MS/MS and database searching Protein identification Full scan MS/MS spectra from doubly charged parent ion m/z 807.2 and matching theoretical sequence identified by database searching Identification of structurally similar aflatoxins Analysis of peptides using CE/MS/MS Gas Chromatography (GC) Gas Chromatography • Mobile phase is gas. Stationary phase can either be solid or non-volatile liquid. • GC involves a sample being vapourised and injected onto the chromatographic column. • Sample is transported through the column by the flow of inert gaseous mobile phase. Gas Chromatography GC: Instrumental components • Carrier gas – Carrier gas must be chemically inert. – Commonly used gases: nitrogen, helium, argon, carbon dioxide. • Sample injection port – Temperature of the sample port is usually about 50°C higher than the boiling point of the least volatile component of the sample. GC: Instrumental components • Columns – Packed columns – Capillary columns • Column temperature – Oven temperature is kept constant for a straightforward separation GC: Instrumental components • Detectors – Concentration dependant detectors: • Signal is related to concentration of solute • Not destroy the sample • Dilution of with make-up gas will lower response – Mass flow dependant detectors • Destroy sample • Signal is related to rate at which solute molecules enter the detector. • Response is unaffected by make-up gas Support Selectivity gases Flame Hydrogen Mass flow Most organic cpds. ionization (FID) and air Thermal Concentrati conductivity Reference Universal on (TCD) Halides, nitrates, nitriles, Electron Concentrati Make-up peroxides, anhydrides, capture (ECD) on organometallics NitrogenHydrogen Mass flow Nitrogen, phosphorus phosphorus and air Hydrogen Flame Sulphur, phosphorus, tin, and air photometric Mass flow boron, arsenic, germanium, possibly (FPD) selenium, chromium oxygen Aliphatics, aromatics, ketones, Photo-ionization Concentrati esters, aldehydes, amines, Make-up (PID) on heterocyclics, organosulphurs, some organometallics Hall electrolytic Hydrogen, Halide, nitrogen, nitrosamine, Mass flow conductivity oxygen sulphur Detector Type Detect Dynami ability c range 100 pg 107 1 ng 107 50 fg 105 10 pg 106 100 pg 103 2 pg 107 Gas Chromatography References • • • • • • • • • • • • http://www.waters.com/ http://www.chemguide.co.uk https://https://www.thermofisher.com Chromatography. The Royal Society of Chemistry. An Introduction to Mass Spectrometry. The University of Leeds. Mass Spectrometry in Biotechnology Gary Siuzdak , Academic Press 1996 SiuzdakBiotechnology” An Introduction to Gel Permeation Chromatography and Size Exclusion Chromatography. Agilent Technologies. Size-exclusion Chromatography of Polymers. Encyclopedia of Analytical Chemistryilent Chromatography for influenza vaccine manufacture: Principles, Equipment and Design. NC State University Basics of LC/MS. Agilent Technologies. Gas chromatography. Sheffield Hallam University. http://teaching.shu.ac.uk/hwb/chemistry/tutorials/chrom/gaschrm.htm