* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 15604-54726-1
History of invasive and interventional cardiology wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Aortic stenosis wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
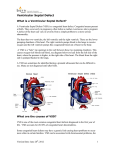
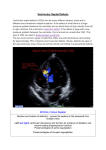
Aortic valve prolapse misdiagnosed as aortic sinus aneurysm in patients with ventricular septal defect: analysis of the echocardiographic findings Guobing Hu 1: MD, Fang Song 2: MD, Xiangming Zhu 1: MD. All authors declare that they have no conflict of interest. This article has been supported by funding from the First Affiliated Hospital of Wannan Medical College Program: Grant No.WK2015ZF. 1: Department of Ultrasound, the First Affiliated Hospital of Wannan Medical College, Wuhu 241001, Anhui, China 2: Department of Radiology, the Number Five People Hospital of Wuhu, Wuhu 241001, Anhui, China Correspondence: Guobing Hu Department of Ultrasound the First Affiliated Hospital of Wannan Medical College Wuhu 241001, Anhui, China Tel: 00865535712869 Fax: 00865535738279 E-mail: [email protected] Aortic valve prolapse misdiagnosed as aortic sinus aneurysm in patients with ventricular septal defect: analysis of the echocardiographic findings Abstract Objective To retrospectively analyze the preoperative echocardiographic findings of ventricular septal defect (VSD) associated with aortic valve prolapse (AVP) in 9 patients who were misdiagnosed as ventricular septal defect asscociated with aortic sinus aneurysm (ASA). Methods Between June 2005 and May 2015, 92 patients were diagnosed as VSD associated with ASA by transthoracic echocardiography (TTE). Intraoperatively, 83 patients were confirmed as VSD associated with ASA, 9 patients were confirmed as VSD associated with AVP. In this paper, we mainly discuss the 9 patients who were misdiagnosed by TTE. All patients were performed with Philip IE33 echocardiograph.6 were males and 3 were females. Results Subarterial VSD was diagnosed in all patients by TTE and confirmed during surgery. Both color and continuous Doppler showed continuous turbulence in the right ventricle outflow tract (RVOT) in all patients. Aneurysm -like protrusions could be detected and diagnosed as AVP by two dimensional TTE in RVOT in all patients. During surgery, all of the aneurysm -like protrusions were confirmed as over enlarged right coronary cusps bulging into RVOT. Conclusions Occasionally, subaterial VSD associated with AVP would be misdiagnosed as subaterial VSD associated with ASA by TTE. To reduce the misdiagnosis rate and provide a more precise preoperative diagnosis, multisection, multi-angle observation of these diseases should be performed. The ultrasonic doctors should also have a thorough hemodynamic knowledge. Key words: aortic valve prolapse, ventricular septal defect, aortic sinus aneurysm, transthoracic echocardiography, misdiagnosis. Introduction Subarterial VSD is frequently associated with AVP. Aortic sinus aneurysm can also be associated with subarterial VSD. Previous studies of these diseases showed that echocardiography has become an useful noninvasive technique for diagnosing subarterial VSD associated with AVP without the need for cardiac catheterization [1], however a certain misdiagnosis rate could be made while using TTE[2], and there is very little literature about the causes of echocardiographic misdiagnosis, therefore we collected 9 patients who were diagnosed as subarterial VSD associated with ASA by TTE but later confirmed as subarterial VSD associated with AVP in surgery. We retrospectively analyze the preoperative echocardiographic findings in the 9 patients and hope that the misdiagnosis rate could be reduced a more accurate preoperative diagnosis can been provided for clinical heart surgical operation. Methods Between June 2005 and May 2015, 92 patients were diagnosed as subarterial VSD associated with ASA by TTE. Intraoperatively, 83 patients were confirmed as subarterial VSD associated with ASA, 9 patients were confirmed as subarterial VSD associated with AVP. In this paper, we mainly discuss the 9 patients who were misdiagnosed by TTE. All patients were performed with Philip IE33 echocardiograph.6 were males and 3 were females. There were 6 women and 3 men, aged between 3 and 45 (mean 16.5). Auscultation revealed continuous murmur along the left sternal border in all patients, TTE was performed in all patients with an IE33 echocardiograph, A transducer of 3, 3.75, 5 was used depending on the patient’s physique and the kind of echocardiograph used. All patients were confirmed with operative findings. The comparison and analysis between the images of TTE and the operative findings were made in the 9 patients. Results All of the 9 patients were diagnosed as subarterial VSD by TTE, and the intro-operative findings confirmed the diagnosis. Both color and continuous Doppler showed continuous turbulence in the RVOT in all patients, aneurysm -like protrusions were detected by two-dimensional TTE in RVOT in all patients. Operative findings confirmed those aneurysm -like protrusions were over enlarged right coronary cusps bulging into RVOT. Due to infective endocarditis, perforation of the right coronary cusp was found in all patients. Other associated congenital heart diseases included double chambered right ventricle, secondary atrial septal defect and patent ductus arteriosus. Aortic regurgitation could be detected in various degrees in all patients by TTE. Comparison between TTE diagnosis and operative findings in the nine patients (table 1) Discussion VSD is one of the most common congenital heart diseases. There are many subtypes of VSD [3], among them, sub-arterial VSD is a unique subtype which is always seen in Asians [4,5]. Different from other types of VSD, sub-arterial VSD is known for low tendency for spontaneous closure as well as progressive AVP and aortic regurgitation due to: “venturi pressure effect” of the left to right shunt and a lack of anatomical support of the aortic valve [6,7]. Open-heart surgery has been advocated as the gold treatment of sub-arterial VSD. The introduction of echocardiography has made it possible to diagnose the location and presence of VSD and aortic valve deformity noninvasively [8]. RCSA is often associated with VSD [9], although we know that the echocardiographic criteria for diagnosis of RCSA include:1) the root of the aneurysm is above the aortic annulus,2) the aneurysm is saccular,3) continuous turbulence and high velocities could be detected by continuous wave Doppler distal to the area of rupture,4) color flow shows mosaic turbulence across the ruptured aneurysm in real time [10]. We could still occasionally misdiagnose AVP as RCSA in subarterial VSD while using TTE. In all patients, the size of subarterial VSD measured by TTE was smaller than that seen in surgery. This is because the prolapsed coronary cusps were found tethered to the VSD margin, overlaid part of the VSD and had reduced the size of the VSD [10]. In addition, the jet of VSD was not parallel to the ultrasound beam [11]. Ultrasonic doctors should be aware of this phenomenon because it is very common. In all 9 patients, the pronounced distortion and translocation of aortic annulus resulting from the lack of anatomical support due to large subarterial VSD made it difficult in judging the exact location of the root of the aneurysm -like protrusions while using two –dimensional TTE. Valve excrescences can always form on the surface of aortic valves as a result of infective endocarditis, they can appear not only in the shape of nodular masses, but also in the shape of long strips [9, 10]. Excrescences could be detected by TTE in all patients, TTE clearly displayed the excrescences floating around VSD, covering the aortic annulus, making it even more difficult to demarcate the root of aneurysm -like protrusions. We suggest that the continuity of the coronary cusp and the root of aneurysm -like protrusion should be carefully observed while the aneurysm -like protrusions are suspected of originating from the prolapsed coronary cusps, in addition, because the coronary cusp which prolapses into RVOT through VSD is always enlarged, its movement is not as stable as that of a normal coronary cusp in cardiac cycles. Perforation of the aneurysm -like protrusions triggered by too thin coronary cusps and infective endocarditis could be detected by TTE in all 9 patients, a continuous turbulent flow which was also the typical characteristics of rupture of ASA could be detected in the RVOT by Continuous-wave Doppler [11], in addition, auscultation revealed a continuous harsh murmur along the left sternal border in all patients, so ultrasonic doctors tended to make the diagnosis of rupture of ASA. We should keep this in mind that the continuous turbulent flow detected in RVOT is not unique to RASA. Apart from the main causes of misdiagnosis mentioned above, other causes including: (1) The irregular shape of VSD, the complex structure around VSD and the associated abnormalities such as double-chambered right ventricle could also affect the diagnosis [12, 13]. (2) Inappropriate gain adjustment of the ultrasound machine, poor imaging of two-dimensional TTE, and anamorphose of color flow signal could also cause misdiagnosis [14, 15]. (3) Due to a lack of skill or understanding of the disease, or limited experience, ultrasonic doctors only observed large VSD and failed to scan the exact location of the aneurysm -like protrusion, Conclusion Sometimes it is very difficult to make an accurate diagnosis of subarterial VSD associated with AVP while using TTE. To reduce misdiagnosis and to provide a precise preoperative diagnosis of these diseases, multisection, multi-angle observation of the aneurysm -like protrusions should been performed and the ultrasonic doctors should also have thorough hemodynamic knowledge. Authors' Contributions Concept/design: Guobing Hu; data analysis/interpretation: Fang Song, drafting article: Xiangming Zhu; critical revision of article: Guobing Hu, approval of article:Guobing Hu, Fang Song, Xiangming Zhu. 1. Ian-Jun G, Xue-Gong S, Ru-Yuan Z, et al: Ventricular septal defect closure in right coronary cusp prolapse and aortic regurgitation complicating VSD in the outlet septum: which treatment is most appropriate? Heart Lung Circ 2006; 15(3):168-171. 2. Cho MS, Jang SJ, Sun BJ, et al: Prognostic implications of initial echocardiographic findings in adolescents and adults with supracristal ventricular septal defects. J Am Soc Echocardiogr 2014; 27(5):965-971. 3. Zhang S, Zhu D, An Q, et al: Minimally invasive perventricular device closure of doubly committed sub-arterial ventricular septal defects: single center long-term follow-up results. J Cardiothorac Surg 2015 ; 15(7): 100-119. 4. Goldberg JF: Long-term Follow-up of “Simple” Lesions—Atrial Septal Defect, Ventricular Septal Defect, and Coarctation of the Aorta. Congenital Heart Disease 2015; 10(3): 466–474. 5. Cheung YF, Chiu CS, Yung TC, etal: Impact of preoperative aortic cusp prolapse on long-term outcome after surgical closure of subarterial ventricular septal defect. Ann Thorac Surg 2002; 73(4): 622-627. 6. Zhao T, Hu J, Yang Y: Anatomic and functional aortic valvuloplasty for correction of aortic valve prolapse in ventricular septal defect with aortic insufficiency. Ann Thorac Surg 2011; 91: 308-310. 7. Chiu SN, Wang JK, Lin MT, et al: Progression of aortic regurgitation after surgical repair of outlet-type ventricular septal defects. Am Heart J 2007; 153(8):336-342. 8. Yoshimura N, Hori Y, Horii Y, et al: Comparison of magnetic resonance imaging with transthoracic echocardiography in the diagnosis of ventricular septal defect-associated coronary cusp prolapse. J Magn Reson Imaging 2010; 32(9):1099-1103. 9. Doll U, Herberg U, Tiemann K, et al: Ruptured sinus of Valsalva aneurysm in two patients with subarterialventricular septal defect. Clin Res Cardiol 2006; 95(6):127-131. 10. Chiu SN, Wang JK, Lin MT, et al: Aortic valve prolapse associated with outlet-type ventricular septal defect. Ann Thorac Surg 2005 ;79(8): 1366-1371. 11. Saleeb SF, Solowiejczyk DE, Glickstein JS, et al: Frequency of development of cuspal prolapse and aortic regurgitation in patients with subaortic ventricular aortic septal defect diagnosed at <1 year of age. Am J Cardiol 2007; 99(7):1588-1592. 12. Suh CH, Yang DH, Kang JW, et al: Demonstration of doubly committed juxta-arterial ventricular septal defect with aortic valve prolapse by cardiac computed tomography. J Cardiovasc Comput Tomogr 2014 ; 8(9):83-94. 13. Devlin PJ, Russell HM, Mongé MC, et al: Doubly committed and juxtaarterial ventricular septal defect: outcomes of the aortic and pulmonary valves. annals Thorac Surg 2014;97: 2134-2140. 14. Choi YJ and Park SW: Characteristics of double-chambered right ventricle in adult patients. Korean J Intern MED 2010; 25(5):147-153. 15. Guobing Hu, Xiangming Zhu, Guojie Li, et al: A case report of double-chambered right ventricle associated with subarterial ventricular septal defect and rupture of right coronary sinus aneurysm. Echocardiography 2015; 32(9):1438-1440. Figure 1. A. Continuous-wave Doppler showed a continuous turbulent flow during both systole and diastole. B. The over enlarged right coronary cusp appeared to be bulbous and bulging into the RVOT, valve excrescence could be displayed in the shape of small masses. C. Color Doppler showed blood flow across large VSD during systole. D.RVOT view displayed perforation of right coronary cusp. Figure 2. A. Continuous-wave Doppler showed a continuous turbulent flow during both systole and diastole. B. The excrescence appeared in the shape of long strips in the RVOT. C. The over enlarged right coronary cusp appeared to be bulbous and bulging into the RVOT, perforation of right coronary cusp could also be displayed. D Color Doppler showed blood flow across large VSD during systole. Figure 3. A. Continuous-wave Doppler showed a continuous turbulent flow during both systole and diastole. B.RVOT view displayed perforation of right coronary cusp. C. The over enlarged right coronary cusp appeared to be bulging into the RVOT, perforation of right coronary cusp could also be displayed. D Color Doppler showed blood flow across large VSD during systole.. RVOT=right ventricular outflow tract; LA=left atrium; RA=right atrium, LV=left ventricle, RV=right ventricle; AVP=aortic valve prolapse. VSD=ventricular septal defect.