* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Nature of Matter
Survey
Document related concepts
Transcript
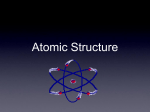
The Chemistry of Life The Nature of Matter Atoms!!! Building Block of Elements Contains a nucleus = center Has Energy Levels surrounding the outside Each atom is distinct for each individual element Atomic Structure The protons and neutrons make up a concentrated core= NUCLEUS The electrons are in constant motion circling the nucleus near the speed of light!! Parts of the Atom Protons: positive charge; nucleus Neutrons: no charge; nucleus Electrons: negative charge, around nucleus in levels or shells Elements Made of the same kind of atoms Cannot be broken down 90 elements occur naturally on earth They can all be found on the PERIODIC TABLE. THE PERIODIC TABLE Reading the Periodic Table Left to Right, Top to Bottom All elements are represented by symbols *They increase in increments of 1 *atomic number= # of protons *atomic mass= protons + neutrons Consider The Following… Atomic # Atomic Mass 27 58.9 Co Element Name Cobalt Atomic Symbol What is an Isotope? Atoms of the same element that have different numbers of NEUTRONS. The same elements will ALWAYS have the same number of protons. Ex: Carbon 12 and Carbon 14 Find The Difference! http://web.visionlearning.com/custom/chemistry/animations/ CHE1.3-an-isotopes.shtml Note and explain the difference between the atoms shown! Link courtesy of vision learning! What is an Ion??? An atom that has either lost or gained an electron from it’s outer most shell and taken on a charge Valence Shell = Outer Most Shell + = loss of an electron - = gain of an electron Ex: Na+, Ca 2+ , Cl - Compounds Two or more elements bonded together Almost all substances in “nature” occur as compounds When elements combine to make compounds, they LOSE their original properties. Ex: H2O, CO2, and HCl Types of Bonds Bonding= 2 or more elements either sharing or stealing electrons Takes place in the OUTER MOST energy level Who you bond with depends on the number of electron vacancies in the valence shell Rules of Molecular Bonding 1. 2. 3. All atoms want to achieve STABILITY. Stability = FULL Valence Shell However many electrons you need or can give away determines “who” you bond with. COVALENT BONDS formed when 2 atoms share electrons in the outer most energy level forming a molecule with NO CHARGE Classic Example: Water!!! IONIC BONDS 2 oppositely charged ions attract each other, forming a neutral bond (canceling each other out) Classic Example: Sodium Chloride = Table Salt For Example Chlorine= 17 electrons 2-first shell 8-second shell 7-third shell (holds 8) so, Cl needs one more e- to be stable SODIUM Na has 11 e-, which means… 2 first shell 8 second shell only 1 in third shell (holds eight) So, Na needs to LOSE one e-!!! Why Do They Bond?