* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmcokinetics in Critical Care
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacogenomics wikipedia , lookup
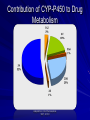
Pharmacokinetics in Critical Care Objectives review basic pharmacokinetic & pharmacodynamic principles apply these principles to scenarios commonly encountered in critical care Pharmacokinetics describes the movement of drugs through the body 4 phases: – absorption – distribution – metabolism – elimination Pharmacodynamics describes the action of a drug once it reaches the target tissue relationship between drug concentration & effect can be used clinically Important Definitions Linear or First-Order Kinetics – constant % of drug eliminated per unit of time – increased drug concentration proportional to increased dose – elimination proportional to drug concentration – shown by most drugs Important Definitions Non-Linear or Zero-Order Kinetics – constant amount (vs %) of drug eliminated per unit of time – drug concentration changes not proportional to dose changes – often a function of metabolic enzyme saturation – eg. alcohol, phenytoin Important Definitions Half-life (t1/2) – time required for half of an administered drug to be eliminated Steady State – rate of drug being administered = rate being eliminated – results in constant serum levels – achieved in 4-5 half-lives – achieved more quickly with loading doses Case 74 yo male admitted to ER with hypoxemic respiratory failure diagnosed with CAP; intubated & transferred to ICU started on: – Ceftriaxone 1 g IV q24h – Azithromycin 500 mg IV q24h Case pt weaned & extubated within 2 days then sent to ward stepped down to levofloxacin 500 mg PO q24h for 7 days total on Day 6, Code 66 is called for respiratory failure – transferred back to ICU – reintubated – antibiotics changed back to IV pt improves quickly & is sent back to ward within 2 days Case What went wrong? – during ICU stay, the patient was started on continuous enteral feeds – when antibiotics were stepped down to oral, no instructions were left to hold the feeds 2 hours pre/post administration – binding interaction ultimately lead to levofloxacin treatment failure Binding Interactions affected classes: – quinolones, phenytoin, tetracyclines often dramatic effects on peak concentration & time to peak worst offender = phenytoin – hold feeds for administration – always order capsules for NG admin; liquid adsorbs more to feeding tubes Other Issues with Drug Absorption ileus: watch for high residuals, usually confirmed radiographically ischemic bowel N/V/D malabsorption/short gut syndrome hemodynamic instability – may impact gut perfusion Advantages of Oral Meds improves patient’s sense of well being minimizes potential for phlebitis or line infections earlier ICU discharge up to 8-fold savings in drug acquisition Case RS, a 48 yo (wt 85 kg) male, presents to ER with 3 day history of cough, dyspnea, & fever initial investigations reveal Sp02 84% & LLL opacity on CXR RS has a positive history for EtOH & currently resides in a homeless shelter empiric antibiotics started for CAP – Ceftriaxone 2 g IV q24h – Azithromycin 500 mg IV q24h Case following intubation & stabilization, RS is admitted to ICU during the first 3 days of admission, he demonstrated clinical improvement & the team was able to wean his vent settings to minimal support on Day 4, his 02 requirements increased, along with increased suction passes, & he developed a new consolidation on CXR antibiotics broadened to Tazocin 3.375 g IV q6h & vancomycin 1 g IV q12h to cover VAP Case as per protocol, a vancomycin pre-level was drawn prior to 3rd dose – level comes back at 4 mg/L Vancomycin Dosing use actual body weight loading dose – helps to attain therapeutic concentrations more quickly – 25-30 mg/kg – be cautious in renal failure maintenance dose – 30-60 mg/kg/day divided q8-12h Vancomycin Monitoring follow trough levels (peaks have limited clinical utility) – to start, measure pre-3rd or pre-4th dose levels (to ensure serum concentration has reached steady state) target levels dependent on tissue penetration at various sites – CNS: 20-25 mg/L – lungs: 15-20 mg/L – skin/soft tissue:10-15 mg/L Back to the Case following the pre-3rd level of 4 mg/L, the team reassesses the dose/interval on rounds – target level = 15-20 mg/L – 60 mg/kg/day x 85 kg = 5100 mg ÷ q8h = 1700 mg – round to nearest 250 mg = 1500 mg q8h new dose pre-3rd level = 18 mg/L Case KM, a 38 yo male (wt 75 kg), presents to ER in status epilepticus intubated en route by EMS & given midazolam 5 mg x 3 doses upon arrival, loaded with phenytoin 1 g & started on propofol infusion @ 50 mcg/kg/min transferred to ICU once stable Case the following morning, propofol was weaned down & turned off that evening, the patient began to experience tonic-clonic seizure activity propofol restarted & kept on overnight phenytoin monitoring: – level comes back following morning at 7 µmol/L (ref range 40-80 µmol/L) – albumin 22 corrected level = 13 µmol/L Case based on this level: – a phenytoin ‘mini-load’ of 500 mg was given – maintenance dose increased to 150 mg q8h EEG showed abatement of seizure activity – propofol again weaned & turned off once awake, extubated & sent to ward repeat phenytoin level: 88 µmol/L (Alb 25) – Corrected = 147 µmol/L Phenytoin Pharmacokinetics non-linear aka Michaelis-Menton kinetics – phenytoin demonstrates saturable metabolism – http://otm.oxfordmedicine.com/content/vol5/issue1/images/large/med-9780199204854-graphic010004.jpeg Impact of Hypoalbuminemia phenytoin is 90% plasma protein bound – normally, only 10% of phenytoin is unbound & able to exert a therapeutic effect changes in serum albumin can drastically change the amount of unbound phenytoin albumin is an acute phase reactant so levels almost always decreased in critical illness Phenytoin Dosing loading dose – 15-20 mg/kg IVPB – max infusion rate = 50 mg/min – always check compatibilities - incompatible with dextrose solutions – Oral: 15-20 mg/kg in 3 divided doses q2-4h maintenance dose – start at 100 mg q8h (4-7 mg/kg/day) IV to PO conversion 1:1 Phenytoin Monitoring time to draw levels – 2-4 hours after IV loading dose (not common) – trough level ~1 h before next dose; first level 3-4 days after initiation (or earlier if seizure activity still present) correcting for albumin – low albumin with CrCl > 25 mL/min Cpcorrected= Cpreported 0.02 x albumin (g/L) + 0.1 – Use 0.01 x albumin + 0.1 for CrCl < 25 mL/min Phenytoin Monitoring free vs. total phenytoin levels – free levels indicate the unbound fraction of phenytoin – ref range: 4-8 µmol/L – useful when: adequate dose & sub-therapeutic level pt with therapeutic level has signs of toxicity pt has known derangement in volume of distribution, eg. critically ill, pregnant, renal failure, cirrhosis, burns Phenytoin: Dose Adjustment do not adjust dose more than once a week nomogram for adjusting phenytoin dose level < 20 increase x 100-200 mg qd level 20-30 increase x 100 mg qd level 31-40 increase x 50 mg qd level>40 increase x 30 mg qd From: Phenytoin Pharmacokinetics Training Manual. Duane Bates. AHS Back to the Case repeat phenytoin level = 88 µmol/L (147) – likely a product of saturable metabolism despite the high corrected level, KM is not demonstrating signs of toxicity – toxicity: nystagmus, ataxia, CNS changes maintenance dose changed from 150 mg IV q8h to 300 mg PO daily. – follow-up levels in therapeutic range Case LB, an 86 yo female (wt 60 kg) admitted to CV ICU following CABG x 2 long bypass pump time due to bleeding arrives on unit heavily sedated: – fentanyl 100 mcg/h – midazolam 5 mg/h decision made to keep at RASS of -2 to -3 until following morning Case next morning, her hemodynamic parameters are stable plan to wean sedation to minimize pressor & ventilation settings fentanyl weaned to 25 mcg/h & midazolam turned off by afternoon: – RASS score still –2 – unable to be extubated due to LOC Case the following day: – LOC starts to improve – RASS score returns to 0 – still requiring ventilatory support the next morning: – Patient passes SBT, has cuff leak, & a reasonable LOC to allow airway protection Volume of Distribution (Vd) describes rate & extent of plasma transfer of a drug determined by several factors: – lipophilicity: increased lipophilicity = increased distribution (including distribution past BBB) – plasma protein binding increased PPB = decreased distribution – drug polarity – tissue blood flow Midazolam Distribution highly lipid soluble, i.e. large Vd easily penetrates BBB accumulates in adipose tissue – leads to creation of depot effect drug continues to leach out of adipose tissue even after the medication is discontinued Case RR, 65 yo male (wt 75 kg), admitted to medicine ward following GI bleed medical history significant only for CAD pre-admission meds: – ASA 81 mg qd – atorvastatin 80 mg qhs – metoprolol 25 mg bid – ramipril 10 mg qd Case urea breath test confirms presence of Helicobacter pylori started on eradication therapy: – amoxicillin 1000 mg bid – clarithromycin 500 mg bid – lansoprazole 30 mg bid Case on Day 4 of antibiotics, RR begins to experience muscle weakness/tenderness on Day 5, he develops flank pain & darkened urine labs show a CK of 8000 U/L managed with supportive care aimed at maintaining adequate urine output Drug Metabolism aka biotransformation process of enzyme-catalyzed changes in drug structure – increases hydrophilicity to promote excretion occurs at many sites in the body – liver, gut, lungs, kidney, brain consists of Phase I & Phase II reactions Consequences of Drug Metabolism Substrate Active Toxic Inactive Non-toxic Metabolite Inactive Non-toxic Active (Pro-drug) Toxic (Reactive Metabolite) Cytochrome P450 Metabolism Phase I oxidative metabolism most common & important of all metabolic pathways – catalyzes metabolism for 60-80% of marketed drugs over 270 gene families identified – 18 known in humans CYP families 1, 2 & 3 most clinically relevant Contribution of CYP-P450 to Drug Metabolism Adapted from: Clin Pharmacokinet 1997; 32:210 Medication Classes Implicated in CYP-Mediated DIs CYP 3A4 CYP 2C9 CYP 2C19 CYP 2D6 CYP 1A2 Substrates Benzos Midazolam Cyclosporine A Tacrolimus CCBs Statins PIs NSAIDs S-warfarin Phenytoin Propranolol Omeprazole Diazepam Codeine SSRIs/TCAs Venlafaxine DM Risperidone Haloperidol Beta-blockers R-warfarin Clozapine Caffeine Theophylline Imipramine Inhibitors Azoles Clarithromycin Erythromycin Cimetidine Grapefruit juice Azoles Ritonavir Azoles Fluoxetine Sertraline Omeprazole Ritonavir Methadone Cimetidine SSRIs (>Paxil) Venlafaxine Fluphenazine Haloperidol Azoles Clarithromycin Erythromycin Cimetidine Grapefruit juice Inducers Phenobarbital Rifampin Dexamethasone Carbamazepine St John’s Wort Phenytoin Phenobarbital Rifampin Phenobarbital Rifampin None reported Phenobarbital Rifampin Phenytoin Omeprazole Smoking www.aafp.org/afp/980101ap/cupp.html Case: Pharmacogenetics LR, 78 yo female (wt 65 kg), admitted to CV ICU following aortic valve replacement 2 hours post-op, she flips into atrial fibrillation with HR in the 120s management – amiodarone 150 mg fast loading dose, then 1 mg/min infusion for 6 h, 0.5 mg/min for 18 h – no beta blockers due to continuing need for pressor support Case: Pharmacogenetics following 24 hours of amiodarone, LR remains in atrial fibrillation, but her pressor support has been weaned off & she’s been extubated team decides to try beta-blockade for rate control – started on metoprolol 12.5 mg po bid – amiodarone continued at 0.5 mg/min Case: Pharmacogenetics following her 3rd dose of metoprolol, LR converts back to sinus rhythm after her next dose, she starts to develop bradycardia with a HR of 45-49 metoprolol is held & amiodarone is converted to oral pt maintains sinus rhythm in the 60s-70s Pharmacogenetics genetic polymorphisms in drug metabolizing enzymes, transporters or receptors can cause clinically relevant effects on efficacy & toxicity of drugs individuals respond differently to drugs there are poor, intermediate, & ultra-rapid metabolizers Pharmacogenetics Back to the Case metoprolol is metabolized by the CYP-2D6 isoenzyme – if LR is a poor metabolizer, metoprolol could accumulate, leading to bradycardia other beta-blockers affected include: – labetalol – carvedilol – propranolol Case EB, 45 yo female (wt 55 kg), who is quadriplegic from long-term care, presents to ER with hypoxic respiratory failure requiring intubation recently hospitalized for cellulitis started on empiric antibiotics: – Tazocin 3.375 g IV q6h – vancomycin 1 g IV q12h Case pre-3rd vanco dose level = 28 µmol/L SCr = 110 µmol/L u/o ~30 mL/h CrCl ~50 mL/min Case How can this level be explained? due to quadriplegia, her baseline SCr = 30 µmol/L so an increase to 110 µmol/L is suggestive of acute renal failure Serum Creatinine creatinine = breakdown product of creatine freely filtered by kidney; acts as a surrogate for glomerular filtration this assumption is based on normal & consistent production of creatinine Serum Creatinine conditions that impact use of SCr as a surrogate for GFR include: – malnourishment – highly catabolic states – muscle wasting: MS/muscular dystrophy – para/quadriplegics – elderly In these groups, always refer to the patient’s baseline SCr when making assumptions about renal function Back to the Case based on the vanco level of 28 mg/L, the following adjustments were made: – 55 kg x 15 mg/kg = 825 mg dose changed to 750 mg & interval to q24h subsequent levels came back in the therapeutic range Case KC, 58 yo male (wt 70 kg) with ESRD secondary to diabetic nephropathy, presents to ER c/o right leg pain with fever (T 39.2) & leukocytosis (WBC 24.4) physical exam reveals a large ulcer on KC’s right foot & initial blood cultures grow gram-ve bacilli he usually has hemodialysis 3 times a week Case empiric antibiotics are initiated as follows: – Tazocin 2.25 g IV q8h (plus top-up prn) – vancomycin 1 g IV (if level < 15 pre-dialysis) due to moderate hypotension, he is transferred to ICU for monitoring decision is made to convert to CRRT to manage hypotension (hoping to avoid pressors) Case the following day on rounds, the clinical pharmacist wonders why the antibiotics have not been adjusted for CRRT Dialysis in Critical Care CRRT: Continuous renal replacement therapy – used in hemodynamically unstable patients – labour-intensive & costly IHD – patients are converted to IHD once hemodynamically stable enough to handle removal of large volumes of fluid Dialysis & Drugs assessment of a drug’s pharmacokinetic profile will indicate how it is impacted by dialysis – look at Vd & PPB – drugs that are dialyzable are cleared more rapidly on CRRT – remember to adjust doses – refer to Critical Care CRRT dosing chart on iweb Dialysis & Drugs remember to re-adjust drug doses once a patient converts back to IHD, otherwise the patient can become toxic resources for dialysis dosing – Clinical pharmacist – Dispensary pharmacist (evening hours) – Lexi-Comp or Micromedex Important Drugs in Renal Disease antibiotics milrinone phenytoin digoxin meperidine, morphine, midazolam