* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mutualisms in a changing world: an evolutionary
Island restoration wikipedia , lookup
Biogeography wikipedia , lookup
Biological Dynamics of Forest Fragments Project wikipedia , lookup
Introduced species wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Habitat conservation wikipedia , lookup
Molecular ecology wikipedia , lookup
Theoretical ecology wikipedia , lookup
Ecogovernmentality wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
Punctuated equilibrium wikipedia , lookup
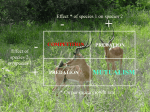
Ecology Letters, (2010) IDEA AND PERSPECTIVE 1 doi: 10.1111/j.1461-0248.2010.01538.x Mutualisms in a changing world: an evolutionary perspective 2 E. Toby Kiers, * Todd M. Palmer, Anthony R. Ives,3 John F. Bruno4 and Judith L. Bronstein5 1 Institute of Ecological Science, Faculty of Earth and Life Sciences, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands 2 Department of Biology, University of Florida, Gainesville, FL 32611, USA 3 Department of Zoology, University of Wisconsin, Madison, WI 53706, USA 4 Department of Marine Science, Abstract There is growing concern that rapid environmental degradation threatens mutualistic interactions. Because mutualisms can bind species to a common fate, mutualism breakdown has the potential to expand and accelerate effects of global change on biodiversity loss and ecosystem disruption. The current focus on the ecological dynamics of mutualism under global change has skirted fundamental evolutionary issues. Here, we develop an evolutionary perspective on mutualism breakdown to complement the ecological perspective, by focusing on three processes: (1) shifts from mutualism to antagonism, (2) switches to novel partners and (3) mutualism abandonment. We then identify the evolutionary factors that may make particular classes of mutualisms especially susceptible or resistant to breakdown and discuss how communities harbouring mutualisms may be affected by these evolutionary responses. We propose a template for evolutionary research on mutualism resilience and identify conservation approaches that may help conserve targeted mutualisms in the face of environmental change. University of North Carolina, Chapel Hill, NC 27599-3330, USA 5 Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ 85721, Keywords Adaptation, climate change, cooperation, corals, dispersal, invasive species, pollination, rhizosphere, selection pressures, species interactions. USA *Correspondence: E-mail: [email protected] Ecology Letters (2010) INTRODUCTION Human activities are driving global environmental degradation at an unprecedented speed and scale (Brook et al. 2008). As the loss of global biodiversity accelerates, biologists are focusing conservation efforts on determining proximate drivers of species loss and identifying means to assure global ecosystem functioning. In doing so, research is revealing that much of the global diversity at stake is underpinned by mutualisms–cooperative interactions among different species (Bascompte & Jordano 2007; Tylianakis et al. 2008; Potts et al. 2010). Every species on earth is involved directly or indirectly in one or more mutualistic partnerships; some are involved in hundreds (Bronstein et al. 2004). Mutualists are central to the survival and reproduction of multitudes of organisms, providing essential ecosystem services, such as pollination (Potts et al. 2010) and seed dispersal (Galetti et al. 2008; Terborgh et al. 2008), and constituting critical components of global carbon and nutrient cycles (Wilson et al. 2009). Many major evolutionary transitions enabling the diversification of life itself have hinged on mutualistic interactions, including the evolution of the eukaryotic cell and the colonization of land by plants associated with fungal mutualists (Bronstein et al. 2004). While the interdependence of mutualists has made possible many evolutionary opportunities, it also carries a cost: because mutualisms can bind multiple species to a common fate, the potential breakdown of these interactions carries the risk of expanding and accelerating the effects of global change and other causes of species extinctions. Recently, case studies have begun to accumulate illustrating the existence of mutualism breakdowns (Table 1). Ocean warming and other local stresses have disrupted partnerships between reef-building corals and their photosynthetic bacterial mutualists, altering the functioning of reef ecosystems (Hoegh-Guldberg et al. 2007). Mutualisms between plants and their pollinators and seed dispersers are being disrupted by habitat loss and fragmentation (Winfree et al. 2009; Potts et al. 2010). Accidental introductions of 2010 Blackwell Publishing Ltd/CNRS 2 E. T. Kiers et al. Idea and Perspective Table 1 Examples of anthropogenic drivers and the ecological and evolutionary responses they modify Each example illustrates the conservation question associated with the mutualism listed, but questions apply broadly to a wide range of threatened mutualisms not listed here. References for footnotes are listed in main text and ⁄ or Appendix S1 references: 1Jones et al. (2008), 2LaJeunesse et al. (2009), 3Stat et al. (2008), 4Yang & Rudolf (2010), 5Hegland et al. (2009), 6Doi et al. (2008), 7Memmott et al. (2007), 8Egerton-Warburton et al. (2007), 9Nijjer et al. (2010), 10Johnson (2010), 11Winfree et al. (2009), 12Potts et al. (2010), 13Murua et al. (2010), 14Eckert et al. (2009), 15Terborgh et al. (2008), 16 Jordano et al. (2007), 17Wolfe et al. (2008), 18Reinhart & Callaway (2006), 19Carey et al. (2004), 20Rodriguez-Cabal et al. (2009), 21Lach (2008), 22 LaJeunesse et al. (2005) and 23Palmer et al. (2008). 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective non-native species and biological invasions are disrupting native mutualisms (Traveset & Richardson 2006), and global nutrient loading is leading to new evolutionary trajectories for mutualistic micro-organisms (Egerton-Warburton et al. 2007). Is a broader crisis involving mutualism breakdown looming? Or are these isolated, atypical examples? Within the past few years, several reviews have examined the relationship between global change and ecological species interactions (e.g., Dunn et al. 2009; Yang & Rudolf 2010; Berg et al. 2010). In one comprehensive study, Tylianakis et al. (2008) synthesized data from 688 published studies to illustrate that global change (e.g., CO2 enrichment, nitrogen deposition, climate, biotic invasions and land uses) is driving sometimes seemingly minor changes in individual interactions, but that these changes can compound, resulting in more profound effects on community structure. Other authors have focused on the ways in which global change has disrupted particular mutualistic systems, notably plant– pollinator interactions (Eckert et al. 2009; Hegland et al. 2009; Winfree et al. 2009), highlighting specific mechanisms (e.g., climate-induced phenological mismatch) that threaten the ecological persistence of partnerships. Much of the discussion of biological responses to global change has focused on observations from the last 50 years, complemented by work projecting changes 50–100 years into the future. In this context, emphasis has been placed on the ecological aspects of mutualisms, such as the mortality of corals due to bleaching and limited fruit set by plants in the absence of pollinator mutualists. Nonetheless, many mutualisms have existed for tens of thousands to many millions of years, exhibiting remarkable persistence and stability. Mutualisms have formed and dissolved over evolutionary time scales, undergoing dramatic shifts in outcome (from mutualism to antagonism), partner identities and specificity (Sachs & Simms 2006). This leads to two crucial questions. First, have mutualisms evolved to be resilient, or flexible, enough to withstand the kinds of anthropogenic disturbances to which they are now being subjected? Second, if they cannot, can mutualists evolve rapidly enough to preserve partnerships over the duration of environmental disturbances acting on decadal time scales? Here, we develop an evolutionary perspective on mutualism breakdown to complement the current ecological perspective. We examine how humans have altered the evolutionary trajectories of mutualisms. We do not examine co-extinction, an ecological process that has been welladdressed in the recent literature (e.g., Bascompte & Stouffer 2009; Dunn et al. 2009). Rather, we survey a wide range of mutualisms and focus on three less-studied responses important to the trajectory of mutualisms (Sachs & Simms 2006): (1) shifts from mutualism to antagonism, (2) evolutionary switches to novel partners and (3) Mutualism breakdown 3 mutualism abandonment (i.e., extinction of the interaction, but not necessarily the partners). We suggest that these processes are among the most widespread and potent, yet least-understood responses of mutualisms to global change; cases in which these responses have altered the evolutionary trajectory of mutualisms are only now being recognized. We follow this with a discussion of the evolutionary factors that may make particular classes of mutualisms especially susceptible or resistant to breakdown, asking whether the consequences of mutualism fate can be predicted. We take particular note of the fact that mutualisms, like species themselves, have evolved in spatially and temporally variable environments (Thompson 2005), and thus may have evolved features conferring resilience that have gone unnoticed. We then scale up to the community level, asking how ecological communities harbouring mutualisms will be affected by these evolutionary responses, and how predictable these effects will be. We propose a research template of evolutionarily focused questions that can be used to investigate potential trajectories of endangered mutualisms. We describe how conservation approaches can be fortified with an evolutionary perspective to help mitigate the impact of global change on mutualisms. EVOLUTIONARY RESPONSES OF MUTUALISMS TO GLOBAL CHANGE Human-driven shifts to antagonism Mutualisms can be markedly dynamic at both ecological and evolutionary time scales, shifting along a natural continuum from mutually beneficial to antagonistic interactions. Changes in biotic and abiotic conditions can tip the balance away from mutualistic exchange and towards exploitative outcomes; a once-beneficial relationship for both partners may become less beneficial or even detrimental, depending on shifting cost:benefit ratios (West et al. 2007). This is a natural outcome of natural selection on mutualist partners, with selection favouring those individuals that abandon a mutualism when costs exceed benefits. Shifts to antagonism by once-mutualistic partners have occurred repeatedly in mutualisms over evolutionary time (Sachs & Simms 2006 and references therein). However, research is revealing that human impacts on global ecosystems can shift the balance of trade (Palmer et al. 2008), driving faster and more farreaching changes than those observed in the past, destabilizing existing partnerships and promoting shifts towards antagonism (Johnson 2010 and references therein). At the level of the biosphere, changes in climatic conditions can create novel niches that facilitate the evolution of antagonism by mutualistic species. Extreme weather patterns have become an increasingly common feature of ecosystems around the world (Allan & Soden 2010 Blackwell Publishing Ltd/CNRS 4 E. T. Kiers et al. 2008). Repeated and prolonged drought episodes in Mediterranean forests have created environmental conditions that select against water-saving benefits conferred by leaf endophyte mutualists. Once-beneficial endophytic leaf partners have been found to adopt growth patterns that allow them to aggressively colonize weakened, dry tree tissue, facilitating their ability to exploit hosts as water becomes limiting. Is this simply a phenotypic shift, or are extreme weather patterns driving selection in this endophyte? Shifts to antagonism could be solely phenotypic, with drought episodes causing morphological and physiological changes that increase pathogenicity. Alternatively, shifts to antagonism could be heritable, with drought episodes favouring more thermophilic, increasingly pathogenic genotypes (Moricca & Ragazzi 2008). Finally, extreme and variable weather could favour the evolution of greater phenotypic plasticity, conferring more flexibility to the fungal partner as persistent droughts become more common and intense. For these Mediterranean forest endophytes, whether such flexibility is the ancestral condition or whether flexibility is itself a trait evolving as a consequence of global warming is unknown. Nonetheless, in systems amenable to the requisite experiments, it would be valuable to investigate the evolution of phenotypic plasticity in response to increasing environmental variances. Shifts to antagonism can likewise be driven if resources once provided by a mutualist partner become readily available from the abiotic environment. In the past 40 years, fertilizer use has increased by 700% worldwide (Foley et al. 2005). Mounting evidence suggests that anthropogenic nutrient deposition may be detrimental to the evolutionary persistence of plant–rhizosphere mutualisms (Johnson 2010). In the short term, nutrient enrichment ameliorates the nutrient limitation that makes rhizosphere mutualists beneficial and can lead host plants to severely decrease or cease resource allocation to their partners. This has been predicted to shift the competitive balance among microbes, favouring the evolution of more aggressive, antagonistic microbial genotypes under increasingly high nutrient conditions (Thrall et al. 2007). Fungal partners such as mycorrhizal mutualists have been shown to adopt hoarding strategies in high fertility soils, for instance allocating more to internal fungal storage at a potential cost to plant hosts (Johnson 2010). Long-term monitoring of mycorrhizal populations at nutrient-enriched sites has revealed increases in less-beneficial strains (Egerton-Warburton et al. 2007 and references therein). Whether changes are ecological (species replacement), evolutionary (individual genetic changes) or represent phenotypic plasticity of existing symbionts is often not determined (see Johnson 2010). Linking antagonism to evolutionary changes in the field can also be problematic because individual mycorrhizal fungal hyphae harbour multiple nuclei. This means that selection in mycorrhizal 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective populations operates at two levels of genetic diversity, among individuals and ÔwithinÕ individuals. Within an individual, some nuclei proliferate under a given nutrient availability, whereas others disappear (Ehinger et al. 2009). Given that rapid genetic divergence co-varies with fitnessrelated traits (such as spore density), this mutualism could be a useful model system for studying processes of genetic erosion and how environmental conditions affect selection for mutualism, among and within individuals. Evolutionary shifts from mutualism to antagonism may also be driven by the loss of species outside the mutualism. All mutualistic interactions are embedded within larger ecological webs. This creates the potential for nonmutualistic species, including predators, parasites (e.g., nectar- and pollen-robbers), and competitors, to strongly influence mutualism evolution. By mediating changes in mutualistsÕ behaviour, network structure and ⁄ or abundance, these species may influence the cost:benefit ratios for mutualisms, potentially shifting their evolutionary outcomes towards antagonism. The importance of external species as drivers of mutualism evolution is still poorly understood. The loss of large herbivores from an African ecosystem has resulted in a shift from mutualism to antagonism in an ant community that typically defends acacia trees from herbivores (Palmer et al. 2008; Fig. 1a). While these changes are mediated over ecological time scales, the potential for evolutionary shifts over longer time scales is clear. In protective mutualisms that have evolved in the context of natural enemies and in which investment in mutualist traits is costly, the loss of those enemies may favour genotypes that invest less in the mutualism (Moraes & Vasconcelos 2009). Extreme differences in life spans of the interacting parties (e.g., ants and trees) create imbalances in the potential for each player to respond evolutionarily to anthropogenically altered environments. As externally wrought changes shift the costs and benefits of the interaction, the more rapidly evolving ant species may be forced to abandon the mutualism if the longlived trees cannot keep pace with these changes. Partner switching In some cases, particularly in specialized mutualisms, anthropogenic change is driving shifting allegiances. For mutualisms exhibiting population-level endemism and specificity, the long-term adaptive capacity of the partnership may be low. Loss of interacting species, alteration of the abiotic environment or other drastic change can drive the formation of novel partner combinations (Bronstein et al. 2004; Sachs & Simms 2006; Wornik & Grube 2010), and lead to evolutionary shifts such as increased generality of interactions (Kaiser-Bunbury et al. 2010). In many cases, partner switching is linked to physiological stress (Table 1). Idea and Perspective Mutualism breakdown 5 (b) (d) (a) (c) (e) Figure 1 Anthropogenic effects drive mutualism breakdowns. (a) Cross-section of an Acacia drepanolobium tree occupied by a non-mutualistic ant partner, Crematogaster sjostedti in Kenya. The tunnels are excavated by a cerambycid beetle whose attacks on trees are actively facilitated by C. sjostedti (photo: Todd Palmer). (b) Diseased colony of Acropora cytherea corals from the Northwestern Hawaiian Islands hosting a clade of parasitic zooxanthellae symbionts reported to colonize coral hosts after stressful bleaching events. White and yellow areas of colony are active disease areas, while blue colouration is healthy host tissue (photo: Michael Stat). (c) Invasive Argentine ants (Linepithema humile) enter flowers and interfere with pollinators of an endangered cactus (Ferocactus viridescens) in California, USA (photo: John Ludka). (d) Coleoptera larva attacks the fruit of Iriartea deltoidea in western Amazonia. Over hunting of seed dispersers has resulted in huge caches of undispersed seeds at parental trees that are vulnerable to attack by various pests (photo: Patricia Alvarez). (e) Community-level disruption. Root tips containing ectomycorrhizal fungi intertwined with roots of Alliaria petiolata (thin white strands), an invasive species of North America shown to facilitate its spread by disrupting mycorrhizal associations (photo: Benjamin Wolfe). Hence, while partner switching certainly occurs naturally within mutualisms, environmental change seems highly likely to increase its frequency (Jones et al. 2008; Hegland et al. 2009). While switching can be evolutionarily adaptive, especially when a speciesÕ present partner is experiencing a serious decline, it is not without danger; mutualists can end up with lower quality partners than they had previously, increasing the risk of extinction. Evolutionary persistence of mutualisms should be favoured, when mutualists are able to select the best partner under a given set of environmental conditions (West et al. 2007). For example, the intensely debated ÔAdaptive Bleaching HypothesisÕ proposes that coral bleaching (i.e., the expulsion of photosynthesizing zooxanthellae partners from their coral reef hosts) reflects active mutualism management, with coral hosts switching to zooxanthellae partners that exhibit increased thermal stress tolerance to warming ocean temperatures (Jones et al. 2008). However, host stress has likewise been linked to outbreaks of opportunistic zooxanthellae partners, which are not necessarily beneficial to hosts (LaJeunesse et al. 2009). In one example, expulsion of mutualistic zooxanthellae during thermal stress increased the susceptibility of corals to fastgrowing symbionts that conferred lower benefits (Stat et al. 2008; Fig. 1b). Importantly, the flexibility afforded to corals able to associate with multiple partners does not guarantee their evolutionary persistence. Using a quantitative genetics approach, Csaszar et al. (2010) found that high heritabilities of functional traits, short clonal generation times and large population sizes allow for rapid thermal adaptation of algal symbionts, but not coral hosts, raising concerns over the adaptability of the interaction to climate change. To date, there is no evidence that high phenotypic variance of symbionts in corals provides greater capacity for evolutionary adaptation than those with low variance. Instead, recent models indicate that shuffling symbionts may increase the capacity of corals to acclimatize, but not necessarily to adapt evolutionarily, to ocean warming (van Woesik et al. 2010). For seasonally dependent mutualisms, partner switching may be the only option as climate change drives mutualists out of synchrony with one another (Yang & Rudolf 2010). Analysis of a remarkable 50-year data set for four Prunus species and a butterfly pollinator revealed that plants are flowering earlier, while the butterflyÕs phenology has 2010 Blackwell Publishing Ltd/CNRS 6 E. T. Kiers et al. remained unchanged (Doi et al. 2008). Evolutionary selection stemming from partner mismatches has the potential to be stronger for pollinators than plants because of their more complete reliance on host-derived nutrition. Survival will strongly depend on the potential for (parallel) adaptation of partners and whether adaptations will be driven mainly by abiotic factors or by the selection pressures plants and pollinators exert on each other (Hegland et al. 2009). Rapid evolutionary responses and reliance on more generalized pollinator associations are key processes that may prevent potentially adverse phenological mismatches. Partner switches may also be forced upon species rather than chosen. New and competitively superior species introduced by humans into a mutualistic guild may become the only available partners for the otherwise stranded mutualist. The invasive Argentine ant (Linepithema humile), a relatively ineffective mutualist, is displacing native ant mutualists around the world that confer crucial pollination, protection and seed dispersal services (Lach 2008; Fig. 1c). In protection mutualisms, Ôpre-adaptationsÕ, such as the ability of L. humile to respond to alarm signals of native ant species, have facilitated the emergence of novel mutualistic associations between many invasive and native species (Mondor & Addicott 2007). However, Argentine ants generally fail to provide adequate mutualistic services to plants, causing significant reductions in fruit and seed set (Blancafort & Gomez 2005). From an evolutionary perspective, these novel interactions have the potential to counter-act pollination and dispersal selection on floral and fruit traits, such as quantity and quality of rewards (Rowles & OÕDowd 2009). Adding a layer of complexity to simple predictions, invasive species can also in some cases act as more beneficial partners, or at least ecologically adequate replacements, compared to native counterparts. The evolutionary consequences of invasive replacement are not well understood. Invasive plants often offer high nectar rewards whose nutritional value to native pollinators (Lopezaraiza-Mikel et al. 2007) may cause their foraging preferences to shift away from native plants (Munoz & Cavieres 2008). This has the potential to lead to reproductive failure or to favour the evolution of new pollination strategies in native plants, such a shift to self-pollination (Eckert et al. 2009). Novel mutualistic relationships with introduced species can compensate for loss of native mutualist extinctions, but not without long-term consequences. Roughly 100 years after its introduction, the avian white-eye, Zosterops japonicus, of the Japanese Bonin Islands has established evolutionarily novel seed-dispersal mutualisms with native plant species (Kawakami et al. 2009). But its introduction may likewise be fuelling the range expansion of invasive plants by contributing to longer distance seed dispersal (Kawakami et al. 2009). Similarly, honeybees, known for their highly adapt 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective able nature, are playing the role of Ôrescue mutualistsÕ by pollinating native plant species following habitat fragmentation and loss of native pollinators (Goulson 2003). However, their spread may be leading to transmission of pathogens and parasites to native organisms and reduced out-crossing rates, resulting in a reduced gene flow and the promotion hybridization between native plants (KaiserBunbury et al. 2010). Under global change there is the strong potential for mutualisms to evolve to become more generalized. The more generalized nature of some mutualisms may provide insurance against the detrimental impacts of any specific environmental disturbance by increasing the chances that the mutualist assemblage contains at least some species that show natural resistance (Bascompte & Stouffer 2009). Although plant species have been shown to be under selection to modify their reproductive traits (e.g., corolla size) to attract the available mutualist community, those with more generalist traits are expected to have even higher probabilities of initial establishment (Kaiser-Bunbury et al. 2010). Just as generalist plants potentially benefit from pollination by a diverse set of pollinators, trees that utilize ÔredundantÕ partners for seed dispersal in the logged forests of the Indian Eastern Himalaya are less vulnerable to human disturbance than those that rely exclusively on hornbill seed dispersal (Sethi & Howe 2009). However, one danger of relying on multiple species is that what may superficially appear to be a redundant mutualist community may actually be structured by niche specialization, with partners providing complementary – not necessarily equivalent – benefits (Stachowicz & Whitlatch 2005). Likewise, the quality of services offered by ÔredundantÕ partner species may differ. Over multiple generations, small differences in partner quality could have strong evolutionary consequences. For example, seed dispersal distance can vary widely within guilds of seed dispersers: in one system, longdistance dispersal events crucial for maintaining genetic structure of tree populations was shown to be provided by only particular subsets of mutualists (Jordano et al. 2007). Loss of these functionally disparate partners has the potential to alter the evolutionary trajectory of the mutualism more than loss of other partner species. If interacting with redundant partners implies some degree of generalization, then one possible outcome of species loss is the potential evolution of more specialized traits in formerly generalized species as they interact with a narrower range of partners. Does this have the potential to back mutualists into an Ôevolutionary cornerÕ as they become specialized, affording them less flexibility to interact with invading or newly ecologically dominant partners? These are key evolutionary questions that need to be asked if we are to understand if and how mutualisms will adapt to global change. Idea and Perspective Mutualism loss In some mutualisms, switching to a novel partner may not be an option. Alternative mutualists are likely to be ecologically and ⁄ or phylogenetically similar to the resident partners and to show concurrent declines in abundance in response to the same environmental disturbance (Rezende et al. 2007). This means that entire assemblages of mutualists can degrade in response to global change (see Appendix S1 references). One possible response to degradation of entire mutualist guilds is that mutualistic interactions are abandoned completely, even if the partners themselves do not go extinct. Evolutionary studies indicate that most mutualists are not locked in an embrace from which they cannot escape (Sachs & Simms 2006). For example, many long-lived mutualists exhibit traits that buffer them against prolonged absences of partners (Bronstein et al. 2004). One illustration, stemming from dramatic pollinator declines, is an apparent evolutionary shift in certain plants away from reliance on biotic pollen vectors towards the use of abiotic pollen vectors (e.g., water or wind) or exclusive self-pollination. For example, several originally insect-pollinated plant lineages have switched to wind- or bird-pollination after colonization of islands, potential due to decreases in available pollinator fauna (Kaiser-Bunbury et al. 2010 and references therein). The evolutionary persistence of mutualism may become less important for some partners as environmental change proceeds. For example, natural occurrence of native myrmecophtye (obligate ant–plant) plant populations devoid of obligate mutualistic ants has recently been noted in a mainland low-elevation site of the Brazilian Cerrado (Moraes & Vasconcelos 2009). Lower herbivore pressure and concurrent selection for increased constitutive defences were named as possible factors favouring mutualism abandonment in these populations (Moraes & Vasconcelos 2009). Likewise, it has been hypothesized that certain grass hosts may ÔencourageÕ the loss of costly fungal endophtye partners by failing to vertically transmit them to subsequent generations (Afkhami & Rudgers 2008). Research is needed to understand the fitness benefits of this purportedly ÔimperfectÕ transmission of mutualists under changing environmental conditions. The ability of some species to readily form and dissolve mutualistic partnerships should theoretically offer a selective advantage in spatially or temporally variable environments irrespective of global change. However, if certain anthropogenic impacts chronically reduce the selective benefits of mutualisms, as may be the case with the effect of global nutrient enrichment on nutritional mutualisms, partnerships may ultimately be abandoned. For example, as soil fertility rises, plants sever their connections to mycorrhizae because Mutualism breakdown 7 the benefits they confer become redundant with an abiotic source that does not require a costly ÔpaymentÕ (Kiers & Denison 2008; Johnson 2010). Many ruderal plant families (such as the Brassicaceae) typically found in nutrient-rich environments have lost their ability to form mutualisms with mycorrhizae (Wang & Qiu 2006), even under low nutrient conditions. With global nutrient enrichment, the evolutionary abandonment of the mycorrhizal mutualism by more plant families is a possibility: evolutionary loss of the Ômycorrhizal conditionÕ has occurred repeatedly in independent lineages, most notably in species colonizing in nutrientrich environments (Wang & Qiu 2006). Nutrient enrichment may likewise drive the loss of particular aquatic mutualisms. In marine systems, planktonic diatoms and dinoflagellates adopt nitrogen fixers as partners to obtain organic nitrogen used for vitamins and nitrogenrich defensive chemistry. However, when oceanic nitrogen is abundant, phytoplankton abandon bacterial partners, suggesting high maintenance costs (Hay et al. 2004). It is unknown how the current substantial changes in global marine nitrogen cycles will modify the ecological persistence of and evolutionary selection for marine N2-fixing mutualisms (Mahaffey et al. 2005), but phylogenetic analyses suggest that partner abandonment is a common route to mutualism breakdown (Sachs & Simms 2006). From the perspective of biodiversity management, mutualism abandonment is a lesser-of-two-evils compared to co-extinction; at least one partner survives, and sometimes both. However, mutualism loss can drive what is known as the Ôempty-forestÕ syndrome in which mutualistic partners are still present, albeit at extremely low densities, but the functional aspects of the mutualism are gone (Redford 1992). The loss of entire mutualist guilds still has the potential to induce major ecosystem-level changes. For example, evolutionary abandonment of mycorrhizal mutualists would mean loss of massive fungal hyphal networks that are critical for global carbon sequestration and soil stabilization (Wilson et al. 2009), while loss of marine N2-fixing mutualists could alter global oceanic nutrient cycles (Mahaffey et al. 2005). The conservation consequences of mutualisms loss must be considered at an ecosystem scale. Do human-related activities consistently have a negative impact on mutualistic interactions? Global change not only offers the potential for mutualism breakdown, but also the potential for mutualism reinforcement. Contrary to doomsday predictions, there are clear cases in which mutualisms show a surprisingly adaptability to global change. In one of the few explicit evolutionary studies of mutualism and global change, range expansion of a protective ant–plant mutualism was accompanied by the 2010 Blackwell Publishing Ltd/CNRS 8 E. T. Kiers et al. Idea and Perspective evolution of more dispersive traits in two ant-associated species (one mutualistic, one parasitic), but not by changes in dispersal or mutualism investment by the tree host. Despite this asymmetry, there was no evidence of destabilization of the symbiosis at the colonization front (Leotard et al. 2009). In other cases, mutualisms under are actually flourishing in anthropogenically altered environments (e.g., Winfree et al. 2009; Appendix S1 references). The reproduction of generalist plant species can be favoured by invasive pollinators (Goulson 2003), while native pollinators can benefit from the higher nutritional rewards offered by invasive plants (Lopezaraiza-Mikel et al. 2007). The mutualism between sea urchins and their nitrogen-fixing endosymbiotic bacteria is thought to facilitate the spread of dramatic urchin barrens (Hay et al. 2004). Successful invasions by plant species are often facilitated by suites of microbial mutualists that significantly increase the growth of the invader in new areas (Appendix S1 references). For instance, plants in the family Leguminosae, which generally depend on nitrogen-fixing Rhizobium either newly adopted or carried with them, are notorious global invaders. Such Ôenhanced mutualism responsesÕ (Reinhart & Callaway 2006), by which the success of non-native species is fuelled by mutualistic partners, have the potential to drive a transformation of a species from relative rarity to superabundance in the introduced ranges. These studies imply that environmental change and disturbance have the potential to reinforce, rather than degrade, mutualistic partnerships. But how often does this occur? In the course of writing this manuscript, we reviewed some 179 studies on the effects of humans on mutualism function and evolution. In Table 2, we present a ÔvotecountingÕ overview that summarizes the number of studies in which anthropogenic effects enhanced or degraded existing mutualistic interactions (see Appendix S1 for references). This exercise was meant to provide a broad snapshot of the current literature – it is by no means a quantitative analysis. While studies of degradation of mutualisms far outweighed the number of studies on mutualism enhancement, the papers we reviewed consistently presented two routes by which mutualisms are reinforced: (1) when new mutualistic relationships form between exotic species and native mutualists leading to a superabundance of the exotic species and (2) when an environmental change or abiotic stress increases the benefits of an existing mutualism (e.g., increased reliance on a microbial mutualism that protects against drought or temperatures increase). However, the most surprising pattern revealed by this Ôvote-countingÕ exercise was that among these 179 studies, only 15 included an empirical evolutionary component, such as a selection analysis (Table 2; Appendix S1 references), further demonstrating the lack of research on evolutionary processes underlying mutualism disruption and reinforcement. On predicting the evolutionary fates of mutualisms Can general evolutionary responses to mutualism breakdown be predicted? Evolutionary trajectories of mutualisms may be better anticipated if we are able to determine the factors mediating their current persistence (Fig. 2). We suggest a focus on three categories of research to increase our ability to predict mutualistic fate: (1) basic knowledge on the relationship between evolutionary shifts and mutualism type, (2) the use of historical information (e.g., the fossil record or phylogenetic patterns) to map past climatic events onto mutualism phylogenies and (3) an increased understanding of the characteristics of resilient mutualisms. Below, we discuss these three approaches. Evolutionary shifts and mutualism type At least for some mutualistic interactions, we might be able to anticipate some general directional evolutionary shifts based on mutualism type. For example, in a food-fortransportation mutualism, the loss of large-bodied seed dispersers in tropical forests and streams has the potential to Table 2 Categorization of 179 empirical studies investigating the effects of human processes (e.g., global warming, habitat fragmentation, exotic species introduction, nutrient enrichment, over-hunting, pollution, urbanization, etc.) on four mutualism types Mutualism type Degraded by human processes Enhanced by human processes Neutral or unaffected Studies counted Number of studies with evolutionarily component Pollination Dispersal Protective Nutritional 42 33 10 31 9 4 5 13 20 1 3 8 71 38 18 52 Total = 179 6 3 1 4 15 (59%) (87%) (56%) (60%) (13%) (10%) (28%) (25%) (28%) (3%) (17%) (15%) Percentage of total for each category is listed in parenthesis. Counts of studies including an evolutionary component (e.g., selection analysis) are listed in last column. 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective Mutualism breakdown 9 Figure 2 Schematic drawing of potential evolutionary trajectories of mutualistic partners and their interactions under anthropogenic change. Factors that drive or determine trajectories are listed in boxes. Positive outcomes for mutualisms persistence are highlighted in green, whereas negative outcomes are highlighted in red. drive selection for small (but not larger) fruits that could still be dispersed by the remaining mutualist community (Galetti et al. 2008; Terborgh et al. 2008; Fig. 1d). If no alternative partners remain, directional selection may eventually drive fruit evolution towards traits favouring wind or gravity dispersal (Guimaraes et al. 2008). A similar evolutionary trajectory could be anticipated with increased selection for abiotic pollination as a result of pollinator declines (but see Harder & Aizen 2010). In contrast, a route of breakdown less common in these food-for-transportation mutualisms would be a shift towards antagonism. Although plants have evolved mechanisms to exploit pollinators (e.g., evolution of deceptive flowers) and vice versa (e.g., nectar robbing) over long-time scales, in the shorter term it is more likely that antagonism would evolve in nutritional and defensive mutualisms. For example, in ant-protection mutualisms, the wide foraging repertoires of ants allow them to feed upon insects themselves, not only on insect-provided rewards. Ant dietary choices are in large part driven by nutritional balances between proteins and carbohydrates. If changing environmental conditions and community composition were to favour a shift towards a more carbohydrate-rich diet, ants associated with rewardproducing insects could be driven to become predators rather than protectors. Historical data A second approach to increase our ability to anticipate future evolutionary shifts would be to map the evolution of mutualisms against major climatic events (e.g., Brady et al. 2006). A major hurdle for this method is that very few mutualisms have a large enough number of independent origins ⁄ losses to be informative about the effect of climatic events on mutualism evolution. Symbiont-harbouring foraminifera have left an imprint in the fossil record of numerous global change events and are known to respond dramatically to environmental changes. Investigations of the susceptibility of symbiont-harbouring vs. symbiont-free taxa to past changes could help form predictions as to how mutualisms will respond to future changes (Hallock 2000). Characteristics of resilient mutualisms Finally, we will sharpen our ability to predict the evolutionary trajectory of mutualisms by asking what characteristics typify mutualisms that are resilient to current anthropogenic change and how these characteristics are likely to shape those trajectories (Table 3). Below, we explore six such characteristics. Lack of strict dependence Mutualisms should be more resilient when species are relatively insensitive to lapses in services provided by their partners (Table 3). Mutualists with asymmetric dependencies (Bascompte & Jordano 2007) or those that can temporarily forgo services (e.g., long-lived plants that can outwait temporary absences of pollinators or seed dispersers, Bronstein et al. 2004) will be more resilient than those whose short-term survival requires consistent partner interactions (e.g., plants that suffer high mortality in the absence of protective ant guards, Palmer 2003). In obligate mutualisms, there are fewer escape routes, and partners are more likely to become trapped and pushed to extinction by their hosts, and vice versa (Dunn et al. 2009). In contrast, facultative mutualisms theoretically offer a flexibility that 2010 Blackwell Publishing Ltd/CNRS 10 E. T. Kiers et al. Idea and Perspective Table 3 Characteristics that increase the resilience of mutualisms under anthropogenic environmental change and the benefits they confer Characteristics of resilient mutualisms Evolutionary benefit Lack of strict dependence Offers flexibility in times of rapid change Rapid evolution Allows partners to respond to changes in the environmental and each other Broad or novel niches Increases the exploitation potential of rapidly changing conditions Strict control over partners Increases the potential for associating with only high quality partners Tolerance Reduces potential costs of partners when shifting into new ecological contexts Protection from environmental variation Engineering of optimal conditions buffers against environmental fluctuations may be crucial in times of rapid change (Bronstein et al. 2004). Rapid evolution A key attribute of a resilient mutualism is the capacity of partners to respond to changes in the environment and each other. Rapid evolution in response to environmental change may protect one or multiple mutualists, thereby protecting the mutualistic interaction. Studies on the rapid evolution of insects and flowering plants have demonstrated that this mechanism can, but does not always, help mutualisms survive (Franks et al. 2007 and references therein). Mismatches in evolutionary rates can limit the potential for synchronized responses (Hegland et al. 2009). Rapid evolution of one partner, especially an obligate mutualist, will increase mutualism resilience by allowing species to adapt to changing environmental conditions. The rapid evolution of Buchnera aphidicola, the obligate endosymbiont that produces heat shock proteins beneficial to its pea aphid host, is an example. Naturally occurring allelic variation in Buchnera generates variation in aphid tolerance to high temperature. Additional heat tolerance is conferred by another, facultative endosymbiont; natural variation in the presence ⁄ absence of this mutualist creates an extragenomic source of evolutionary variation that can lead to rapid heat shock adaptation (Harmon et al. 2009). These facultative symbiotic interactions in insect hosts are analogous to horizontally transmitted genes in bacteria; both facilitate the immediate introduction of novel capabilities from foreign sources, allowing species to adapt in novel ways to changing conditions (Oliver et al. 2010). However, while such examples are not unique, constraints on rapid evolution may limit its role in protecting species against environmental disturbances. Broad or novel niches Partnerships that increase an individualÕs ability to exploit new niches or broaden a partnerÕs tolerance to changing conditions are likely to be highly successful under rapid environmental change. The ability of fungal endophytes to 2010 Blackwell Publishing Ltd/CNRS confer heat, drought and ⁄ or disease tolerance may contribute to the survival of some plant hosts in increasingly high-stress environments (Appendix S1 references). The microbial gut populations of insects can facilitate their hostÕs ability to colonize novel host plants, for example, by aiding in key plant detoxification steps (Janson et al. 2008). Indeed, the acquisition of bacterial gut symbionts is thought to have driven, or at least facilitated, the evolution of herbivory in ants, opening up a novel feeding niche (Russell et al. 2009). However, costs and benefits of hosting partners are often context-dependent, making it difficult to predict ultimate outcomes. Erwinia, the symbiotic gut microbe of the western flower thrips, can be a mutualist or an antagonist depending upon the composition (leaves vs. pollen) of the hostÕs diet (de Vries et al. 2004). If the thripsÕ diet is altered by environmental change, the gut mutualism has the potential to shift, either becoming increasingly important for exploitation of novel niches or deviating into antagonism. Strict control over partners Resilience may also be promoted when partners maintain strict control over their mutualists. In African fungusgrowing termites, the strict propagation of single variants of their fungal symbiont guarantees exclusive symbiont association, reducing opportunities for the evolution of partner cheating (Aanen et al. 2009). Sanctions against less-mutualistic partners have been demonstrated in many systems, including some legume–rhizobial mutualisms (Kiers & Denison 2008), cleaner–fish mutualisms (Bshary & Grutter 2002) and obligate pollination mutualisms (Goto et al. 2010). Any mechanism that increases the potential for associating with high quality partners will likely facilitate a mutualismÕs persistence under anthropogenic change (Kiers & Denison 2008). However, as noted above, a lack of strict dependence on particular partners will also be important. Tolerance An increased tolerance to short-term costs of mutualists has the potential to benefit partners as mutualisms reorganize under global change. Tolerance strategies can facilitate the Idea and Perspective maintenance of mutualisms by reducing potential costs caused by partner dynamics shifting in new contexts (Edwards 2009). Tolerance strategies will, for example, permit longer lived mutualists, such as ant–plant Acacia species, to be affected less by the immediate impacts of partnering with any single shorter lived ant partner species (Palmer et al. in press). Tolerance to short-term costs, especially under fluctuating environmental extremes such as those predicted under global change, has the potential to translate into greater, or longer term benefits when integrated over a hostÕs lifetime. Protection from environmental variation Some mutualisms are extremely old, such as the 50-millionyear-old mutualism between leaf-cutter ants and the fungus they cultivate. One attribute thought to confer resilience in this mutualism is the way in which the interaction is protected against extreme environmental variation. Ants can engineer optimal environmental conditions, typically by sequestering fungal gardens from the surrounding environment. This is thought to buffer against environmental fluctuations and contribute to the robustness of the mutualism (Mueller et al. 2005). A three-pronged approach that utilizes information from these broad categories (i.e., mutualism type, phylogenies mapped against disturbance, and traits conferring mutualism resilience) will significantly increase our predictive power. For mutualisms involving pairs of linked species, this approach could initially only focus on the evolutionary trajectory of two interacting partners (Fig. 2). However, the evolutionary fates of most mutualisms will depend on their interactions at a larger community scale. Therefore, it will be necessary to address mutualism evolution in the context of the community in which mutualists occur. Evolutionary responses magnified at the community level In ecological communities, most mutualisms involve large networks of species (Bascompte & Stouffer 2009), posing challenges for disentangling evolutionary responses. How will evolution of individual partners affect the response of communities of mutualists to global change? This question is remarkably hard to answer because it depends not only on the genetic variation within species that sets the potential for evolutionary responses (Eckert et al. 2009), but also the complex selective forces that propagate through the ecological pathways of the mutualism network (Murua et al. 2010). Understanding the evolutionary responses of all partners, even those involved in diffuse, indirect interactions (e.g., Fig. 1e), are needed to predict community-level consequences. A major challenge is the high likelihood that global change will produce Ôno-analogue communitiesÕ dominated Mutualism breakdown 11 by novel environmental conditions and mutualistic assemblages that have no current or past equivalents (Williams & Jackson 2007). Global change is driving the development of unique environmental conditions, especially in low latitudes where temperatures will be higher than those seen over the course of most organismsÕ recent evolution. Change in other environmental parameters such as precipitation and seasonality are predicted to create environmental niches not currently existing on earth (le Roux & McGeoch 2008). In one projection, over half of California will be occupied by novel assemblages of bird species by 2070 (Stralberg et al. 2009). Novel niches are likely to result in unanticipated and unpredictable combinations of species, with little, if any, shared evolutionary history. How will mutualisms arise or disintegrate as such communities assemble? Although generalities are impossible to draw, some insight into the overall functioning of the novel communities in which mutualisms are embedded may be gained by more closely studying present-day no-analogue communities: extant systems already dominated by exotic species, such as grasslands, rivers, lakes and estuarine ecosystems. In at least some of these cases, mutualisms readily form between pairs or groups of invasive species, a process that has been termed Ôinvasional meltdownÕ (Simberloff & Holle 1999). Although these systems appear to operate normally, very little work has examined mutualism functioning as the number of inhabitants lacking an evolutionary history together increases (but see Aizen et al. 2008). A second possibility is that mutualistic interactions might act to increase community integrity under global change. Phylogenetic analyses have revealed that facilitative interactions among plant species (i.e., Ônurse effectsÕ) of the past have been important in shaping contemporary plant communities (Valiente-Banuet et al. 2006). Will mutualistic interactions likewise be a glue that maintains the communities in which they are embedded? These types of investigations will be crucial as communities reorganize on a new playing field of altered interactions. Below, we discuss the key questions that should be asked in studies of vulnerable mutualisms facing global change. The questions are aimed at disentangling evolutionary responses and guiding conservation efforts. Template for investigating the evolution of mutualism breakdown Developing a single comprehensive theoretical framework for predicting mutualistic fate in a community context is not realistic. Mutualisms are context-dependent and highly heterogeneous, especially with regard to how tightly interacting partners are bound, what commodities are being exchanged, and how the interactions affect partner fitness. Even for geographically isolated communities of mutualists, 2010 Blackwell Publishing Ltd/CNRS 12 E. T. Kiers et al. understanding the ecological process of co-extinction is difficult because of linkages in mutualistic webs (Bascompte & Stouffer 2009). The challenges for predicting evolutionary processes, such as mutualism abandonment, switching and reorganization may be even greater, especially in the temporal and spatial context thought to be important for the evolution of some mutualisms. For example, the geographic mosaic theory of co-evolution (Thompson 2005) envisions spatio-temporally varying hotspots of coevolution that consist of semi-isolated populations undergoing strong co-evolutionary selection. Demonstrating the evolutionary processes underlying geographic mosaics is a challenge, and by extension, it will be similarly difficult to study the spatio-temporal patterns of co-evolution of mutualisms under current environmental changes. Nonetheless, evolutionary changes at the population (rather than species) level can and should be observed. A major goal in studies of mutualisms should be to anticipate how mutualisms respond to anthropogenic change, determine how these responses could alter ecosystem services, and develop effective countermeasures to be implemented over a time frame of tens to hundreds of years. This likely necessitates an evolutionary-focused approach for many systems. Importantly, our focus should not be limited to protecting specific species – depending upon the species, this may or may not be seen as a critical goal – but should also include preserving the functioning of mutualisms in an ecosystem context. What is needed is a forward-looking scheme, one that incorporates both ecological and evolutionary perspectives. Below, we propose a template of four essential questions that should be asked in studies of mutualisms facing global change; the questions traverse a broad range of biological and temporal scales, and are aimed at guiding interactionbased conservation efforts. At what scale should we aim to conserve the mutualistic community? The future evolutionary trajectory of any mutualism depends not only on the responses of interacting mutualists to global change, but also on the dynamics of the broader communities in which these mutualists are embedded. As such, our conservation efforts may need to encompass both mutualists and the species that influence their densities. For example, increased frequency of heat shocks (shortduration periods of high temperature) may reinforce the mutualism between pea aphids and their secondary endosymbionts, as discussed before. However, changes in aphid abundance will also depend on the presence and behaviours of predator species that may augment or diminish benefits of the mutualism (Harmon et al. 2009; Fig. 3a). Thus, it is critical to determine the scale (i.e. what species are included) at which mutualist communities are targeted for conservation initiatives. 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective When subjected to anthropogenic forces, how do changes in the abundances of mutualists alter the structure of mutualist networks? Mutualism networks are often viewed as static entities, yet changes in the abundances of constituent species can by themselves alter major interaction pathways within networks (Fig. 3b). For example, pollinators are mutualists of plants but may also be competitors with each other. If the abundance of one pollinator species is suppressed by an anthropogenic change, then others may experience novel selection pressures or even competitive release, increasing in density and ultimately maintaining strong pollination benefits to plants. This type of change in the strengths of indirect interactions within guilds of mutualist partners in networks, while poorly documented, needs to be considered in any conservation analysis. Do mutualists change strategies in the face of anthropogenic change? Understanding the strategies behind partner responses, such as coral expulsion of zooxanthellae or shifts from mutualism to exploitation in mycorrhizal communities (Fig. 3c), requires explicit study of the evolutionary responses to changes in costs and benefits under anthropogenic influences. Research is needed into the anthropogenic forces that trigger the evolution of exploitation strategies common to mutualisms that otherwise differ widely in their characteristics. What is the evolutionary context of the mutualism? Many mutualisms are ancient, others relatively young. Evolutionary history might reveal the range of variability a mutualism has experienced and survived. Conversely, past patterns of evolutionary change may point to potential future changes. The prevalence of nested mutualist networks in natural systems (Bascompte & Jordano 2007) and the ability of some partners to successfully abandon mutualistic partnerships might reflect evolutionary (and co-evolutionary) responses to past environmental variability (Fig. 3d). With the caveat that the past does not always predict the future, studying the processes facilitating rapid evolution and other resilience characteristics may provide clues to predicting mutualism survival. PERSPECTIVES: CHALLENGES FOR THE CONSERVATION OF MUTUALISMS While we know of no ongoing mutualism-focused conservation effort that fully addresses the questions of our template, there are conservation programs that hold promise for generating the right types of data. The USA National Phenology Network, a partnership between federal agencies and the academic community, is a monitoring initiative focused on phenology of plants and animals. As data can be collected at the scale of ecological communities, over Idea and Perspective Mutualism breakdown 13 (a) (b) (c) (d) Figure 3 Template of four questions to investigate mutualism breakdown. (a) The prevalence of facultative endosymbionts conferring tolerance to heat shocks in pea aphids is predicted to increase in response to climate change, but changes in aphid communities will likewise depend on changes in predator attack rates, entangling dynamics of mutualist and predator–prey interactions. (b) An anthropogenic disturbance suppresses the abundance of one pollinator leading to the competitive release of another. Pollination benefit remains the same as the plant shifts dependency to the second pollinator (orange lines). Dependency of the pollinator on the plant (red lines) does not change. (c) Hypothetical curve illustrating how increases in abiotic resources for a plant partner can select for exploitative strategies by mycorrhizal partners. (d) Phylogenetic reconstruction of the broad-scale co-evolution of fungus-growing ants (left), their fungal cultivar (middle), and the garden parasite Escovopsis (right) (courtesy of Cameron Currie). multiple generations, the program has the potential to reveal an exceptionally broad diversity of linkages among species and even to serve as an early warning system for when connections begin to break down. On an ecosystem scale, ReefBase, a global information sharing system for coral reefs, and NEON, the US-based National Ecological Observational Network, hold promise as data generators and repositories for evolution studies. However, it is unlikely that broad-based monitoring can successfully identify subtle evolutionary changes within networks; this requires knowledge of how species interact, not just their relative abundances. Furthermore, as species evolve, interactions change quantitatively but also qualitatively, requiring more in-depth study. Modelling approaches are needed that incorporate longer term empirical studies of partner survivorship and mortality in areas with intact vs. altered assemblages. In this way, we can generate more robust predictions linking breakdown to long-term consequences for mutualistic networks. Effective conservation strategies will also require a critical look at how one can extrapolate local-scale effects to larger scales, including global ones. Likewise, how and when can ecological effects be extrapolated to an evolutionary time scale? For example, if facultative, generalist mutualists 2010 Blackwell Publishing Ltd/CNRS 14 E. T. Kiers et al. switch to relying upon less beneficial but more widely available partners, will they evolve greater specialization, such that even if threatened species come to increase once more in abundance, their mutualists will have been permanently lost to them? These are crucial questions that need to be tackled. Although it is unrealistic to expect detailed ecological and evolutionary studies on a majority of mutualisms, key case studies are essential to serve as guides to the range of evolutionary responses. The survival of mutualisms, and the ecosystems in which they reside, depends upon maintaining a strong focus on the evolution of interacting species. ACKNOWLEDGEMENTS This work was funded by an NWO ÔVeniÕ grant (ETK), NSF 0313737(ARI) and NSF DEB-0444741(TMP). We thank Stuart West, Jordi Bascompte, Ford Denison, Miranda Hart, Floortje Bouwkamp for table design and Janine Mariën for figure design, and three anonymous referees for very constructive comments. REFERENCES Aanen, D.K., Licht, H.H.D., Debets, A.J.M., Kerstes, N.A.G., Hoekstra, R.F. & Boomsma, J.J. (2009). High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science, 326, 1103–1106. Afkhami, M.E. & Rudgers, J.A. (2008). Symbiosis lost: imperfect vertical transmission of fungal endophytes in grasses. Am. Nat., 172, 405–416. Aizen, M.A., Morales, C.L. & Morales, J.M. (2008). Invasive mutualists erode native pollination webs. PLoS Biol., 6, 396–403. Allan, R.P. & Soden, B.J. (2008). Atmospheric warming and the amplification of precipitation extremes. Science, 321, 1481–1484. Bascompte, J. & Jordano, P. (2007). Plant-animal mutualistic networks: the architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst., 38, 567–593. Bascompte, J. & Stouffer, D.B. (2009). The assembly and disassembly of ecological networks. Philos. Trans. R. Soc. B., 364, 1781–1787. Berg, M.P., Kiers, E.T., Driessen, G., van der Heijden, M., Kooi, B.W., Kuenen, F. et al. (2010). Adapt or disperse: understanding species persistence in a changing world. Glob. Change Biol., 16, 587–598. Blancafort, X. & Gomez, C. (2005). Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol.-Int. J. Ecol., 28, 49–55. Brady, S.G., Sipes, S., Pearson, A. & Danforth, B.N. (2006). Recent and simultaneous origins of eusociality in halictid bees. Proc. R. Soc. B., 273, 1643–1649. Bronstein, J.L., Dieckmann, U. & Ferrière, R. (2004). Evolutionary Conservation Biology. Cambridge University Press, Cambridge. Brook, B.W., Sodhi, N.S. & Bradshaw, C.J.A. (2008). Synergies among extinction drivers under global change. Trends Ecol. Evol., 23, 453–460. 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective Bshary, R. & Grutter, A.S. (2002). Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav., 63, 547–555. Carey, E.V., Marler, M.J. & Callaway, R.M. (2004). Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecol., 172, 133–141. Csaszar, N.B.M., Ralph, P.J., Frankham, R., Berkelmans, R. & van Oppen, M.J.H. (2010). Estimating the potential for adaptation of corals to climate warming. PLoS ONE, 5, 8. Doi, H., Gordo, O. & Katano, I. (2008). Heterogeneous intraannual climatic changes drive different phenological responses at two trophic levels. Clim. Res., 36, 181–190. Dunn, R.R., Harris, N.C., Colwell, R.K., Koh, L.P. & Sodhi, N.S. (2009). The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. Lond. B, 276, 3037–3045. Eckert, C.G., Kalisz, S., Geber, M.A., Sargent, R., Elle, E., Cheptou, P.O. et al. (2009). Plant mating systems in a changing world. Trends Ecol. Evol., 25, 35–43. Edwards, D.P. (2009). The roles of tolerance in the evolution, maintenance and breakdown of mutualism. Naturwissenschaften, 96, 1137–1145. Egerton-Warburton, L.M., Johnson, N.C. & Allen, E.B. (2007). Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecol. Monogr., 77, 527–544. Ehinger, M., Koch, A.M. & Sanders, I.R. (2009). Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytol., 184, 412–423. Foley, J.A., DeFries, R., Asner, G.P., Barford, C., Bonan, G., Carpenter, S.R. et al. (2005). Global consequences of land use. Science, 309, 570–574. Franks, S.J., Sim, S. & Weis, A.E. (2007). Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. USA, 104, 1278–1282. Galetti, M., Donatti, C.I., Pizo, M.A. & Giacomini, H.C. (2008). Big fish are the best: seed dispersal of Bactris glaucescens by the pacu fish (Piaractus mesopotamicus) in the Pantanal, Brazil. Biotropica, 40, 386–389. Goto, R., Okamoto, T., Kiers, E.T., Kawakita, A. & Kato, M. (2010). Selective flower abortion maintains moth cooperation in a newly discovered pollination mutualism. Ecol. Lett., 13, 321–329. Goulson, D. (2003). Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst., 34, 1–26. Guimaraes, P.R., Galetti, M. & Jordano, P. (2008). Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE, 3, e1745. Hallock, P. (2000). Symbiont-bearing foraminifera: harbingers of global change? Micropaleontology, 46, 95–104. Harder, L.D. & Aizen, M.A. (2010). Floral adaptation and diversification under pollen limitation. Philos. Trans. R. Soc. B., 365, 529–543. Harmon, J.P., Moran, N.A. & Ives, A.R. (2009). Species response to environmental change: impacts of food web interactions and evolution. Science, 323, 1347–1350. Hay, M.E., Parker, J.D., Burkepile, D.E., Caudill, C.C., Wilson, A.E., Hallinan, Z.P. et al. (2004). Mutualisms and aquatic community structure: the enemy of my enemy is my friend. Annu. Rev. Ecol. Evol. Syst., 35, 175–197. Idea and Perspective Hegland, S.J., Nielsen, A., Lazaro, A., Bjerknes, A.L. & Totland, O. (2009). How does climate warming affect plant-pollinator interactions? Ecol. Lett., 12, 184–195. Hoegh-Guldberg, O., Mumby, P.J., Hooten, A.J., Steneck, R.S., Greenfield, P., Gomez, E. et al. (2007). Coral reefs under rapid climate change and ocean acidification. Science, 318, 1737– 1742. Janson, E.M., Stireman, J.O., Singer, M.S. & Abbot, P. (2008). Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution, 62, 997–1012. Johnson, N.C. (2010). Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol., 185, 631–647. Jones, A.M., Berkelmans, R., van Oppen, M.J.H., Mieog, J.C. & Sinclair, W. (2008). A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. R. Soc. B., 275, 1359–1365. Jordano, P., Garcia, C., Godoy, J.A. & Garcia-Castano, J.L. (2007). Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl. Acad. Sci. USA, 104, 3278–3282. Kaiser-Bunbury, C.N., Traveset, A. & Hansen, D.M. (2010). Conservation and restoration of plant-animal mutualisms on oceanic islands. Perspect. Plant Ecol., 12, 131–143. Kawakami, K., Mizusawa, L. & Higuchi, H. (2009). Re-established mutualism in a seed-dispersal system consisting of native and introduced birds and plants on the Bonin Islands, Japan. Ecol. Res., 24, 741–748. Kiers, E.T. & Denison, R.F. (2008). Sanctions, cooperation, and the stability of plant-rhizosphere mutualisms. Annu. Rev. Ecol. Evol. Syst., 39, 215–236. Lach, L. (2008). Argentine ants displace floral arthropods in a biodiversity hotspot. Divers. Distrib., 14, 281–290. LaJeunesse, T.C., Smith, R.T., Finney, J. & Oxenford, H. (2009). Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ÔbleachingÕ event. Proc. R. Soc. B., 276, 4139–4148. Leotard, G., Debout, G., Dalecky, A., Guillot, S., Gaume, L., McKey, D. et al. (2009). Range expansion drives dispersal evolution in an equatorial three-species symbiosis. PLoS ONE, 4, 11. Lopezaraiza-Mikel, M.E., Hayes, R.B., Whalley, M.R. & Memmott, J. (2007). The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecol. Lett., 10, 539–550. Mahaffey, C., Michaels, A.F. & Capone, D.G. (2005). The conundrum of marine N-2 fixation. Am. J. Sci., 305, 546–595. Mondor, E.B. & Addicott, J.F. (2007). Do exaptations facilitate mutualistic associations between invasive and native species? Biol. Invasions, 9, 623–628. Moraes, S.C. & Vasconcelos, H.L. (2009). Long-term persistence of a Neotropical ant-plant population in the absence of obligate plant-ants. Ecology, 90, 2375–2383. Moricca, S. & Ragazzi, A. (2008). Fungal endophytes in Mediterranean oak forests: a lesson from Discula quercina. Phytopathology, 98, 380–386. Mueller, U.G., Gerardo, N.M., Aanen, D.K., Six, D.L. & Schultz, T.R. (2005). The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst., 36, 563–595. Munoz, A.A. & Cavieres, L.A. (2008). The presence of a showy invasive plant disrupts pollinator service and reproductive Mutualism breakdown 15 output in native alpine species only at high densities. J. Ecol., 96, 459–467. Murua, M., Espinoza, C., Bustamante, R., Marin, V.H. & Medel, R. (2010). Does human-induced habitat transformation modify pollinator-mediated selection? A case study in Viola portalesia (Violaceae). Oecologia, 163, 153–162. Nijjer, S., Rogers, W.E., Lee, C.T.A. & Siemann, E. (2008). The effects of soil biota and fertilization on the success of Sapium sebiferum. Appl. Soil Ecol., 38, 1–11. Oliver, K.M., Degnan, P.H., Burke, G.R. & Moran, N.A. (2010). Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol., 55, 247– 266. Palmer, T.M. (2003). Spatial habitat heterogeneity influences competition and coexistence in an African acacia ant guild. Ecology, 84, 2843–2855. Palmer, T.M., Stanton, M.L., Young, T.P., Goheen, J.R., Pringle, R.M. & Karban, R. (2008). Breakdown of an ant-plant mutualism follows the loss of large herbivores from an African Savanna. Science, 319, 192–195. Palmer, T.M., Doak, D.F., Stanton, M., Bronstein, J.L., Kiers, E.T., Young, T.P., Goheen, J.R. & Pringle, R.M. (2010). Synergy of multiple partners, including freeloaders, increases host fitness in an ant-plant mutualism. Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1006872107. Potts, S.G., Blesmeijer, J.C., Kremen, C., Neumann, P., Schweiger, O. & Kunin, W.E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol., 25, 345–353. Redford, K.H. (1992). The Empty Forest. Bioscience, 42, 412–422. Reinhart, K.O. & Callaway, R.M. (2006). Soil biota and invasive plants. New Phytol., 170, 445–457. Rezende, E.L., Lavabre, J.E., Guimaraes, P.R., Jordano, P. & Bascompte, J. (2007). Non-random coextinctions in phylogenetically structured mutualistic networks. Nature, 448, 925–U6. le Roux, P.C. & McGeoch, M.A. (2008). Rapid range expansion and community reorganization in response to warming. Glob. Change Biol., 14, 2950–2962. Rowles, A.D. & OÕDowd, D.J. (2009). New mutualism for old: indirect disruption and direct facilitation of seed dispersal following Argentine ant invasion. Oecologia, 158, 709–716. Russell, J.A., Moreau, C.S., Goldman-Huertas, B., Fujiwara, M., Lohman, D.J. & Pierce, N.E. (2009). Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proc. Natl. Acad. Sci. USA, 106, 21236–21241. Sachs, J.L. & Simms, E.L. (2006). Pathways to mutualism breakdown. Trends Ecol. Evol., 21, 585–592. Sethi, P. & Howe, H.F. (2009). Recruitment of hornbill-dispersed trees in hunted and logged forests of the Indian Eastern Himalaya. Conserv. Biol., 23, 710–718. Simberloff, D. & Holle, B.V. (1999). Positive interactions of nonindigenous species: invasional meltdown? Biol. Invasions, 1, 21–32. Stachowicz, J.J. & Whitlatch, R.B. (2005). Multiple mutualists provide complementary benefits to their seaweed host. Ecology, 86, 2418–2427. Stat, M., Morris, E. & Gates, R.D. (2008). Functional diversity in coral-dinoflagellate symbiosis. Proc. Natl. Acad. Sci. USA, 105, 9256–9261. Stralberg, D., Jongsomjit, D., Howell, C.A., Snyder, M.A., Alexander, J.D., Wiens, J.A. et al. (2009). Re-shuffling of species with 2010 Blackwell Publishing Ltd/CNRS 16 E. T. Kiers et al. climate disruption: a no-analog future for California birds? PLoS ONE, 4, 8. Terborgh, J., Nunez-Iturri, G., Pitman, N.C.A., Valverde, F.H.C., Alvarez, P., Swamy, V. et al. (2008). Tree recruitment in an empty forest. Ecology, 89, 1757–1768. Thompson, J.N. (2005). The Geographic Mosaic of Coevolution. University of Chicago Press, Chicago, IL, USA. Thrall, P.H., Hochberg, M.E., Burdon, J.J. & Bever, J.D. (2007). Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol. Evol., 22, 120–126. Traveset, A. & Richardson, D.M. (2006). Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol. Evol., 21, 208–216. Tylianakis, J.M., Didham, R.K., Bascompte, J. & Wardle, D.A. (2008). Global change and species interactions in terrestrial ecosystems. Ecol. Lett., 11, 1351–1363. Valiente-Banuet, A., Rumebe, A.V., Verdu, M. & Callaway, R.M. (2006). Modern quaternary plant lineages promote diversity through facilitation of ancient tertiary lineages. Proc. Natl. Acad. Sci. USA, 103, 16812–16817. de Vries, E.J., Jacobs, G., Sabelis, M.W., Menken, S.B.J. & Breeuwer, J.A.J. (2004). Diet-dependent effects of gut bacteria on their insect host: the symbiosis of Erwinia sp and western flower thrips. Proc. R. Soc. Lond. B, 271, 2171–2178. Wang, B. & Qiu, Y.L. (2006). Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza, 16, 299–363. West, S.A., Griffin, A.S. & Gardner, A. (2007). Evolutionary explanations for cooperation. Curr. Biol., 17, R661–R672. Williams, J.W. & Jackson, S.T. (2007). Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ., 5, 475–482. Wilson, G.W.T., Rice, C.W., Rillig, M.C., Springer, A. & Hartnett, D.C. (2009). Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal 2010 Blackwell Publishing Ltd/CNRS Idea and Perspective fungi: results from long-term field experiments. Ecol. Lett., 12, 452–461. Winfree, R., Aguilar, R., Vazquez, D.P., LeBuhn, G. & Aizen, M.A. (2009). A meta-analysis of beesÕ responses to anthropogenic disturbance. Ecology, 90, 2068–2076. van Woesik, R., Shiroma, K. & Koksal, S. (2010). Phenotypic variance predicts symbiont population densities in corals: a modeling approach. PLoS ONE, 5, 8. Wornik, S. & Grube, M. (2010). Joint dispersal does not imply maintenance of partnerships in lichen symbioses. Microb. Ecol., 59, 150–157. Yang, L.H. & Rudolf, V.H.W. (2010). Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett., 13, 1–10. SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article: Appendix S1 List of references for Table 2. As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited and typeset. Editor, Jordi Bascompte Manuscript received 1 June 2010 First decision made 5 July 2010 Manuscript accepted 15 September 2010