* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Franck-Hertz - University of Utah Physics
Three-phase electric power wikipedia , lookup
Electrical ballast wikipedia , lookup
Power engineering wikipedia , lookup
Electrical substation wikipedia , lookup
History of electric power transmission wikipedia , lookup
Resistive opto-isolator wikipedia , lookup
Current source wikipedia , lookup
Voltage regulator wikipedia , lookup
Power electronics wikipedia , lookup
Electrical grid wikipedia , lookup
Switched-mode power supply wikipedia , lookup
Buck converter wikipedia , lookup
Surge protector wikipedia , lookup
Opto-isolator wikipedia , lookup
Stray voltage wikipedia , lookup
Vacuum tube wikipedia , lookup
Cavity magnetron wikipedia , lookup
Photomultiplier wikipedia , lookup
Voltage optimisation wikipedia , lookup
Alternating current wikipedia , lookup
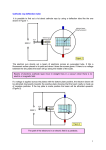
PHYS 3719 - Fall 2014 The Franck-Hertz Experiment NOTE: Part of this material is taken from the PASCO manual; the apparatus used is structurally identical to Pasco, but labeling is different. PASCO’s opaque nomenclature for the various currents and voltages has been changed. Introduction The Franck-Hertz experiment allows you to demonstrate via electrical measurements that electronic energy levels in atoms are quantized. You will also be able to measure the first ionization energy of mercury as well as investigate the correlation between the mean free path of electrons transiting mercury vapor and the current-voltage characteristics of the tube. Experimental Setup Required Equipment SE-9640 Franck-Hertz Tube (Hg) SE-9641 Franck-Hertz Oven Power supplies to provide the following electrical needs: - Filament Voltage, VF, 5 - 6.3 VDC (HP 6126A), supplies current to heat cathode - Accelerating voltage, VGC, 0-30 VDC (Keithley 617, output to LabView) - Opposing voltage, VAG, ≈1.5 VDC (D-cell) Thermometer (to 200° C) Ammeter with sensitivity to 10 pA (Keithley 617, programmed via LabView) Shielded cable with a BNC and Triax connectors DMMs to monitor voltages across filament, from the grid to the cathode and from anode to grid. A simplified diagram of the Franck-Hertz experiment is shown in Figure 1. In an oven-heated vacuum tube containing mercury vapor, electrons are emitted by a heated cathode, and are then accelerated toward a grid that is at a potential VGC relative to the cathode. Just beyond the grid is an anode, which is at a slightly lower potential (i.e. negative with respect to the grid) than that of the grid. If the accelerated electrons have sufficient energy when they reach the grid, some of them will pass through and will reach the anode. They will be measured as anode current IA, by the ammeter. If the electrons don't have sufficient energy when they reach the grid, they will be slowed by VAG, the potential difference between grid and anode, and will fall back onto the grid. Whether the electrons have sufficient energy to reach the anode depends on three factors: the accelerating potential VGC, the opposing potential VAG, and the nature of the collisions between the electrons and the gas molecules in the tube. NOTE: The above description is somewhat over-simplified. Due to contact potentials, the total energy gain of the electrons is not quite equal to eVGC. Therefore, VGC, the potential difference measured for the first current minimum, will be somewhat higher than the actual first ionization energy of mercury. However, the contact potentials are a constant in the experiment, so at successive current minima VGC will always be a multiple of the actual ionization energy. Check this: is the energy of the first current minimum the same as the difference between subsequent minima? Figure 1. Simplified circuit diagram of the Franck-Hertz experiment. About the Franck-Hertz Apparatus (Much of the following is from the PASCO manual) The SE-9640 Franck-Hertz tube is a three-electrode tube with an indirectly heated, oxidecoated cathode, a grid and an anode. The distance between the grid and the cathode in the Pasco apparatus is 8 mm, which is claimed to be large compared with the mean free path of the electrons at normal experimental temperatures (180°C). This ensures a high collision probability between the electrons and the mercury vapor molecules. The distance between the grid and the anode is small to minimize electron/gas collisions beyond the grid. - Estimate the cathode-grid and grid-anode distances in the Neva apparatus you are using and comment on the previous statement based on your calculations of electron mean free path length as a function of oven temperature (below). 2 The tube contains a drop of highly purified mercury. A 10 kΩ current limiting resistor is permanently incorporated between the connecting socket for the accelerating voltage and the grid of the tube. This resistor protects the tube in case a main discharge strikes it when excessively high voltage is applied. For normal measurements the voltage drop across this safe resister may be ignored, because the working anode current of the tube is less than 5 μA. - Calculate the voltage drop across the safety resister under typical conditions you use; compare it to other voltages relevant to your experiment. The tube is mounted to a plate which mounts, in turn, onto one wall of the SE-9641 FranckHertz oven (Figure 2). 3 Anode Grid Oven thermometer Heated cathode Oven heater: vaporizes the mercury Figure 2. Tube vacuum used in the experiment. The SE-9641 Franck-Hertz Oven is a 400 watt, thermostatically controlled heater used to vaporize the mercury in the Franck-Hertz tube*. * Unfortunately, the “thermostatic control” is relatively poor; hence we will set the thermostat to its maximum value and supply the oven heater with a constant voltage from a Variac. The oven temperature – Variac voltage correlation is posted on the wall behind the experimental setup. 4 Caution: Even if you are familiar with the experiment, please read the section Important Cautions and Tips [in the Appendix] before turning on the equipment. A few simple rules can save you the cost of a blown experiment! Instrumentation and Wiring The Keithley 617 Electrometer has two functions: (1) it is programmed via Labview to supply the accelerating voltage from the cathode to the grid (0-30V) and (2) it measures the current into the anode. It outputs both of these values to the computer, which tabulates current as a function of [ramping] voltage, the “answer”. It measures the current by means of a coaxial cable with a BNC connector to the Franck-Hertz cell and Triax connector to the 617. The general layout of all electronic components is shown in Figure 3. The voltage output from the Keithley 617 electrometer is via a pair of blue “Pomona” [brand name] patch cords with banana plugs, as shown in Figure 4. A close-up of the Franck-Hertz faceplate, showing all connection points is given in Figure 5. Note that the sheath of the BNC cable from the electrometer is internally tied to ground, a fact not illuminated by the faceplate drawing (Figure 5). You may confirm this with a DMM. Figure 3. Complete setup of the Frank-Hertz experiment. 5 Figure 4. Ponoma patch cord with banana plugs. Figure 5. Close-up of the Franck-Hertz faceplate. One of the Hewlett Packard 3466A DMMs measures this voltage. How is it connected? A 1.5 V dry cell supplies the retarding voltage between the grid and the anode, and is connected between the grid and the ground terminal, the objective of this voltage is to sharpen the minima between peaks by removing from the anode current those electrons that have lost energy in inelastic collisions and therefore have less than 1.5 eV of energy left. A second Hewlett Packard 3466A DMM measures this voltage. For comparison, do at least one measurement of I-V characteristics with this supply disconnected. Did it actually perform as claimed? 6 Experimental Procedure Collecting Data You will be measuring the current from the grid to the anode as a function of accelerating voltage VGC, or the voltage from the cathode to the grid. The basic procedure for the FranckHertz experiment is straightforward: 1. Heat up the tube to approximately 170°C. 2. Apply the heater (Filament) voltage VF, to the cathode (wait 90 seconds for the cathode to heat). 3. Apply an opposing voltage VA, (approximately 1.5 volts) between the grid and the anode. 4. Slowly raise the accelerating voltage (between the cathode and the grid, VGC) from 0 V to about 30 V. Monitor the tube current to locate the potentials at which the current drops to a minimum. You will not need to change VGC yourself; this process has been programmed for you. Open “keithleyI-V”, which should be located on the desktop of the computer. This program will plot the anode current against accelerating voltage, as well as produce a file containing the data collected, which will be 2 columns of ASCII text (one of voltage and the other of current). On the LabView screen, you will notice several parameters. Adjusting them is fairly self-explanatory. The easiest way to organize your data is to first create a folder. Open this folder, select the pathname for the folder and copy it into the text box located under the “File Path” heading in LabView. Then under “data file” you may change the name of the file to best describe the particular trial you are conducting, e.g. 5Vf,180C(2) for the parameters VF = 5 V, T = 180°C, trial 2. Alternatively you can generate a table with sequential numbers for file names and the values of appropriate operating parameters in each column of the table. The data file can then be opened in e.g. Notepad or Excel, and manipulated with data analysis software (e.g. Origin, Kaleidagraph, Excel, etc.). The Hewlett Packard 6216A DC power supply supplies current to heat the filament, which is directly attached to the cathode. The power supply output connects to points H and K on the oven. Is the polarity of the connections important? Explain. If you set the voltage to a large value, the current knob controls the current in the circuit; if you set the current to a large value, the voltage knob controls the voltage applied to the filament. The Keithley 169 DMM monitors the power supply output voltage; the current output of the HP 6216A current supply is measured by the meter on the supply itself (when the switch on the front is correctly set). Issues to be addressed 1) What is the contribution of the filament current to the experiment? What role does it play in the testing of your model of what’s going on in the Franck-Hertz experiment? Is the exact current critical? Run the experiment twice with voltages in the range 5.0 – 6.0 V or current in the range 200 – 230 mA. Set the oven temperature to be in the range 150-200°C. Do your data support your model of the role of the filament current? 7 2) What is the contribution of oven temperature to the experiment? What role does it play in testing your model of the Franck-Hertz experiment? Is the exact temperature critical? Run the experiment several times with temperatures in the range 150-200°C. Use a filament voltage within 5.0-6.0 V. What effect did you expect temperature to have on the experiment? Do your data support the model of the role of the oven temperature? 3) With your understanding of the role of both factors in testing the model, i.e. the filament voltage/current and oven temperature, run the experiment again selecting a value for the filament voltage between 5.0 and 6.0 V or a filament current between 200-230 mA. The temperature should be set within the range 150-200°C. What is the uncertainty in the anode current that is measured by the electrometer, the “answer”? How will you decrease your uncertainty? What methods can you use to determine the peak voltage? What is your uncertainty in the peak voltage? What is your measurement, with uncertainty, of the lowest excitation energy of mercury? 4) For each of the power supplies you are using, discuss whether the polarity with which you connect them is important. 5) Except at the very lowest applied voltages, the current you measure with the electrometer is always negative. Explain the significance of this sign. 6) If you invert the sign of the current, it is a lot clearer to describe the maxima and minima on your plot. Explain what is going on at a maximum and at a minimum. (What physics causes the I-V curve to exhibit a maximum or minimum?) Should you be measuring the differences between maxima or minima? 7) What parameter contributes the maximum to the uncertainty on your results? What can be done to improve your precision? 8) Using the value you obtained for the excitation energy, what wavelength would you expect the emitted light to have? Can you see this light? Is it harmful? 9) Explain why your results support or refute the model being tested. Appendix I (Optional for points extra credit) Let’s do a sanity check on some numbers here by estimating the mean free path length of electrons between collisions with mercury atoms and see how it compares to our apparatus dimensions. From kinetic gas theory, the mean free path of gas molecules is given by m = 1/(σn), where m is the mean free path length for molecules, σ is the collision cross-section for interactions between molecules and n is the molecular density in the gas where molecules are colliding. [You can find this expression in the kinetic gas theory section of any text on Thermodynamics; in this case, from F. W. Sears, Thermodynamics, Addison Wesley, Reading MA 8 (1953)]. Remember the ideal gas law: PV = nRT. But n in the expression for 𝜆𝑚 is really n/V, so 1/n is really RT/P. Here R is the Universal Gas Constant, which becomes Boltzmann’s Constant since it refers to molecules rather than moles, T is the absolute temperature and P is the pressure. The collision cross-section can be estimated as simply πr2, where r is the atomic radius, given in the Sargent-Welch periodic table as 1.57 x10-10 m. The vapor pressure of mercury as a function of temperature we can calculate from the generating function given by Kubaschewski, Evans and Alcock, Metallurgical Thermochemistry, Pergammon (1967). log10P(T) = A/T + B log10 (T) + CT + D where D = 10.355, A = -3305, B = -0.795 and C = 0; the result, P(T), is in Torr. There could be an additional correction based on Paschen’s Law: buried in the middle is the claim that since electrons are a lot smaller than molecules (and certainly don’t interact like hard spheres) the mean free path length for electrons is greater than that for molecules by a factor of “about 5.64”. Unfortunately this presents you with a major dilemma: a search of the “conventional literature” leads one to observe that Paschen’s Law deals with dielectric breakdown voltages in gases; no reference has yet been located supporting this “magic” factor of “about 5.64”. So, it is now your responsibility to research the literature on the mean free path of electrons in vapors like mercury, compare what you find to your data and discuss the correlation or lack thereof. In any event, stuff all the information above into an Excel sheet and calculate the mean free path length for electrons in mercury vapor at temperatures of 25, 140, 150, 180 and 200°C and any others you may find useful. Note that there are two temperature dependences in the mean free path: the linear one in the density expression and the “exponential” one in the vapor pressure equation. Sanity check: the Sargent-Welch table gives the boiling point of mercury as 357°C; does your generating function give a reasonable confirmation of this? This example will give you lots of opportunities to convert units, which physicists spend a lot of time doing. Why are we doing this? Compare your calculated values of the mean free path length for electrons at typical operating temperatures to apparatus dimensions given in the handout. What happens at room temperature? Is there a big difference between 150°C and 180°C? Appendix II Important information from the PASCO Manual – Please read before using the Franck-Hertz Apparatus: Whether you are performing the experiment using the Control Unit or using separate power supplies, the following guidelines will help protect your students and the equipment. They will also help you get good results. To Avoid Burns: The outside of the Franck-Hertz Oven gets very hot. Do not touch the oven when it is operating, except by the handle. To Protect the Oven: 9 Be sure the power to the oven is ac and is equal to the rated voltage for the oven. A dc power supply or excessive ac power will produce arcing that will damage the bimetal contacts of the thermostat. To Protect the Tube: Always operate the tube between 150°C and 200°C – Never heat the tube beyond 205°C. Always use a thermometer to monitor the oven temperature. The thermostat dial gives the temperature in °C, but the reading is only approximate. Turn the oven and allow the tube to warm up for 10-15 minutes (to approximately 170°C) BEFORE applying any voltages to the tube. Explanation: When the tube cools after each use, mercury can settle between the electrodes, producing a short circuit. The mercury should be vaporized by heating before voltages are applied. When possible, do not leave the tube in a hot oven for hours on end, as the vacuum seal of the tube can be damaged by outgassing metal and glass parts. If the tube is left in a hot oven for a lengthy period of time, heat the cathode for approximately two minutes and then apply an accelerating potential of approximately 5 volts to the grid before turning off the oven. This will prolong the life of the cathode. To Ensure Accurate Results: Use a shielded cable to connect the anode of the tube to the amplifier input of the Control Unit. After heating up the tube in the oven, apply the heater voltage to the cathode, and allow the cathode to warm up for at least 90 seconds before applying the accelerating voltage and making measurements. Minimizing Ionization Ionization of the mercury gas within the tube can obscure the results of the experiment and, if severe, can even damage the tube. To minimize ionization, the tube temperature should be between 150°C and 200°C and the accelerating voltage potential (between the cathode and the grid) should be no more than 30 V. Even if ionization is not severe enough to damage the tube, the positive mercury ions will create a space charge that will affect the acceleration of the electrons between the cathode and the grid. This can mask the resonance absorption that you are trying to investigate. Ionization is evidenced by a bluish-green glow between the cathode and the grid. In fact, if ionization occurs, the side of the grid facing the cathode will have a blue-green coating and the cathode will have a bright blue spot on its center. If this happens, lower the accelerating potential and check the tube temperature before proceeding. Causes and dangers of ionization: If the tube temperature is too low, the mercury vapor pressure will be low, and the mean free path of the electrons in the tube will be excessive. In this case, the accelerated electrons may accumulate more than 4.9 eV of kinetic energy before colliding with mercury atoms. This can lead to ionization of the mercury gas which can increase the pressure inside the tube and damage the vacuum seal. 10 If the tube temperature if too high, ionization can occur due to interactions between the Mercury ions themselves. Again, pressure will be excessive and the tube can be damaged. If the accelerating voltage is too high, the electrons can still gain excessive energy before striking mercury atoms, even if the temperature is correct and the same problem can occur. Reading Material [1] PASCO manual (available in the teaching lab: South Physics building, Rm. 306). [2] A.C. Melissinos and J. Napolitano, Experiments in Modern Physics, Academic Press, MA (2003), Section 1.3. 11