* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download HW205 Unit 2
Academy of Nutrition and Dietetics wikipedia , lookup
Diet-induced obesity model wikipedia , lookup
Food politics wikipedia , lookup
Obesity and the environment wikipedia , lookup
Food studies wikipedia , lookup
Food choice wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
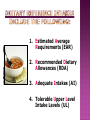
How are supplements regulated? Melissa Dengler, ND, HFS, CLC Understand how the FDA is involved in supplement regulation. Understand what DSHEA is and why it is important. Learn what the difference is between RDAs, DRIs and UL vs. concept of “supplement dosage”. Understand Good Manufacturing Practices. Learn what to look for in choosing a quality supplement. What is a dietary supplement? A dietary supplement is a product that contains vitamins, minerals, herbs or other botanicals, amino acids, enzymes, and/or other ingredients intended to supplement the diet. The U.S. Food and Drug Administration has special labeling requirements for dietary supplements and treats them as foods, not drugs. What four (4) conditions must be met for the product to qualify as a supplement? Product intended to supplement the diet and that contains one or more of the following: vitamins, minerals, herbs or other botanicals, or amino acids Intended to be taken in tablet, capsule, powder, softgel, gelcap, or liquid form Not represented for use as a conventional food or as a sole item of a meal or the diet Labeled as being a dietary supplement. FDA DSHEA GMP FDA DSHEA GMP The first set of nutrients standards in the US originally called Recommended Dietary Allowances (see William’s pg.15) Developed to address the poor nutrition status of WWII recruits Information gathered from expert scientists who studied nutrient needs. Needs are set high above the recommended range to meet the needs of most healthy people. Recommendations scientific research. are made based on 1. Estimated Average Requirements (EAR) 2. Recommended Dietary Allowances (RDA) 3. Adequate Intakes (AI) 4. Tolerable Upper Level Intake Levels (UL) Copyright 2005 Wadsworth Group, a division of Thomson Learning Copyright 2005 Wadsworth Group, a division of Thomson Learning 1. Meet nutritional needs. 2. Avoid nutrient deficiencies. 3. Prevent nutrient toxicity. Given the definition of RDAs, do you think that most people in America are “healthy”? How do we define this? How many people fall in to the “healthy” category? Estimated Energy Requirement Acceptable Macronutrient Distribution Ranges Carbohydrate: 45% - 65% Fat: 20% - 35% Protein: 10% - 35% Copyright 2005 Wadsworth Group, a division of Thomson Learning Copyright 2005 Wadsworth Group, a division of Thomson Learning Specific claims about food and health are allowed. An approved list of health claims. Foods bearing health claims must contain 20% or less the daily value of: Fat (13g), Cholesterol (60mg), Saturated fat (4g) and Sodium (480mg) OR 20% or more of the daily value Calcium (160 mg) Claims made without FDA approval Cannot make statements about disease. E.g. “Improves memory”, “slows aging” Where do you see the role of structure and function claims to help support health? Red Flags of Nutrition Quackery Satisfaction guaranteed Marketers may make generous promises, but consumers won’t be able to collect on them. Quick and easy fixes Even proven treatments take time to be effective. Natural Natural is not necessarily better or safer; any product that is strong enough to be effective is strong enough to cause side effects. One product does it all No one product can possibly treat such a diverse array of conditions. Time tested Such findings would be widely publicized and accepted by health professionals. Paranoid accusations And this product’s company doesn’t want money? At least the drug company has scientific research proving the safety and effectiveness of its products. Personal testimonials Hearsay is the weakest form of evidence. Meaningless medical jargon Phony terms hide the lack of scientific proof. They are regulated as foods, not drugs, so there could be quality issues in the manufacturing process. Supplements can interact with prescribed or over-the-counter medicines, and other supplements. "Natural" does not necessarily mean "safe" or "effective." Consult your health care provider before starting a supplement, especially if you are pregnant or nursing, or considering giving a supplement to a child. Reading: Read Chapters 1 and 2 in William’s Essentials of Nutrition and Diet Therapy and read two articles by the U.S. Food and Drug Administration. Further Notes: Review the Further Notes Discussion: Discuss whether supplements should be regulated by the FDA and the consequences of regulation. Also answer the question about structurefunction claims. Project: You will keep a food journal for one week and analyze the nutrients. You will then write a report indicating where you were deficient Does anyone have any questions about the material covered today?