* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture14Plants-L-type
Survey
Document related concepts
Transcript
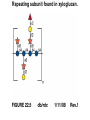
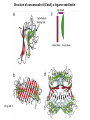
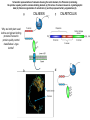
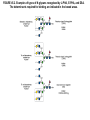
Essentials of Glycobiology May 12th., 2008 Ajit Varki Lecture 14 Chapter 22. Viridiplantae Chapter 29. L-type Lectins Chapter 45: Antibodies and Lectins in Glycan Analysis General Questions for Lecture 14 Why are recombinant mammalian glycoproteins generated in plants immunogenic? Compare the structures of glycoglycerolipids in plants, Lipid A in bacteria, and glycosphingolipids in animals Consider possible functions for L-type plant lectins present in the seeds of leguminous plants. Why are both plant seed lectins and glycan binding proteins involved in protein quality control classified as L-type lectins? What are the advantages and disadvantages of using monoclonal antibodies versus plant lectins for determining the presence or absence of glycans in a preparation? What are important controls when using lectins or anti-glycan antibodies to determine the presence or absence of a glycan in a tissue, on a cell, or in a mixture of glycans? Propose methods to use a monoclonal antibody to a glycan determinant for the isolation a mutant cell line deficient in the expression of the glycan? Types of N-glycans found in plants. Why are recombinant mammalian glycoproteins generated in plants immunogenic? Processing of N-glycans in the plant secretory system. Only those events that are unique to plants are shown in detail. Most abundant plant galactolipids. Compare the structures of glycoglycerolipids in plants, Lipid A in bacteria, and glycosphingolipids in animals Examples of Animal Glycosphingolipids. Compare the structures of glycoglycerolipids in plants, Lipid A in bacteria, and glycosphingolipids in animals Lipid A from Bacteriae Compare the structures of glycoglycerolipids in plants, Lipid A in bacteria, and glycosphingolipids in animals Model of the primary cell wall (type I) found in most flowering plants (except grasses). Cellulose microfibrils are embedded in a hemicellulose (e.g., xyloglucan) and pectin matrix. Repeating subunit found in xyloglucan. Schematic structure of pectin showing the three main pectic polysaccharides: homogalacturonan (HG), rhamnogalacturonan I (RG-I), and rhamnogalacturonan II (RG-II). A region of substituted galacturonan, known as xylogalacturonan (XGA), is also shown. Glycans from fungal and plant cell walls that elicit plant defense responses. Structure of concanavalin A (ConA), a legume seed lectin. Fig.29.1 Comparison of the subunit structures of soybean agglutinin (left) complexed with a pentasaccharide containing Galβ1-4GlcNAc-R and human galectin-3 (right) complexed with lactose Both lectins display a related β-barrel configuration. Three-dimensional structure of a legume lectin (PNA) monomer showing the four loops involved in sugar binding: loops A, B, C, and D. The bound sugar (lactose) is shown as a ball-and stick model. Calcium and manganese ions are required for ligand binding. Fig.29.3a Sequence alignment of loops A–D in legume lectins. The size of binding-site loop D and monosaccharide specificity show an explicit correlation. Monosaccharide specificity and number of gaps are indicated at the right. Key residues are highlighted in blue and highly conserved residues have been indicated with an asterisk. Consider possible functions for L-type plant lectins present in the seeds of leguminous plants. Fig.29.3b Schematic representation of calnexin showing the lectin domain, the P domain (containing the proline repeats), and the calcium-binding domain (a). Structure of calnexin based on crystallographic data (b). Domain organization of calreticulin (c) and its proposed tertiary organization (d). Why are both plant seed lectins and glycan binding proteins involved in protein quality control classified as L-type lectins? Examples of N-glycans recognized by concanavalin A (ConA) from Canavalia ensiformis and Galanthus nivalis agglutinin (GNA). FIGURE 45.2. Examples of types of N-glycans recognized by L-PHA, E-PHA, and DSA. The determinants required for binding are indicated in the boxed areas. Examples of types of glycan determinants bound with high affinity by different plant and animal lectins. The determinants required for binding are indicated in the boxed areas. Examples of types of glycan determinants bound with high affinity by different plant lectins. The determinants required for binding are indicated in the boxed areas. Examples of different glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. What are the advantages and disadvantages of using monoclonal antibodies versus plant lectins for determining the presence or absence of glycans in a preparation? Additional examples of different glycan antigens recognized by specific monoclonal antibodies. The antigens have the structures shown within the boxed area and are named as indicated. Propose methods to use a monoclonal antibody to a glycan determinant for the isolation a mutant cell line deficient in the expression of the glycan? Examples of different uses of plant and animal lectins and antibodies in glycobiology. Many plant and animal lectins are multivalent, and antibodies are always multivalent. They can be used to detect glycan structures in all of the formats shown. What are important controls when using lectins or antiglycan antibodies to determine the presence or absence of a glycan in a tissue, on a cell, or in a mixture of glycans? An example of the use of different immobilized plant lectins in serial lectin affinity chromatography (SLAC) of complex mixtures of glycopeptides.