* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry in Context: Chapter 3:The Chemistry of Global Warming
Survey
Document related concepts
Solar air conditioning wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
History of chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Molecular dynamics wikipedia , lookup
History of molecular theory wikipedia , lookup
Transcript
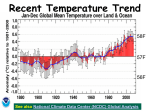
Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Earth • 78% N2 and 21% O2 • Less than 1% other gases • Average temperature = 15 °C • Expected temperature based on solar • • radiation and distance from sun -18 °C 33 °C warmer than expected; hence no frozen oceans and life flourishes. Water vapor and CO2 play a role in trapping solar radiation in the form of heat. Venus • Atmospheric pressure is 90x that of Earth • 96% CO2 and sulfuric acid clouds • Average temperature = 450 °C • Expected temperature based on solar radiation • and distance from sun is 100 °C Possibility of CO2 absorbing infrared radiation and trapping solar heat in the atmosphere. History of Global Warming • Fourier (1800): proposed “hothouse” or • • “greenhouse” effect. Tyndall (1860): demonstrated CO2 and H2O absorb heat Is there any correlation among the following 3 known facts. – CO2 absorbs heat – Concentration CO2 in atmosphere is increasing – Earth’s average temperature is NOT constant History of Global Warming-2 • Early atmosphere: 1000x CO2? • CO2 trapped heat that warmed up Earth to allow life to develop 3 billion years – primitive plants (e.g. cyanobacter) carry out photosynthesis with light-capturing chlorophylls – 6 CO2 + 6 H2O + hν → C6H12O6 + 6 O2 – Availability of O2 allowed the evolution of animals – Earth temperature is 10-15 °C higher 100 million years ago. Fig. 3.2 on Page 101 Evidence for CO2 Warming • Drilled cores from ocean floors – Microorganisms → temperature. – Magnetic field in sediment → time • Antarctic ice cores provided ratios of (2H/1H) and CO2 levels for past 160 millennia. – Light 1H2O evaporates faster than heavy 2H2O, leading to the enrichment of heavy water in the ocean relative to the atmosphere. – During years of warmer temperatures, more heavy water escapes to the atmosphere that return to Earth as snow or rainfall; hence, higher 2H/1H implies higher temperature. Fig. 3.2 Page 101 Energy Balance Figure 3.4 page 103 Outer Space = -270 °C Earth = 15 °C • ~ 84% heat radiated by Earth is absorbed by gases in atmosphere • Re-radiated back to Earth in the form of “GREENHOUSE EFFECT”. • Greenhouse gases include CO2 , H2O, CH4, and others are increase in concentration, leading to >84% heat returned to Earth, thereby raising the Earth’s average temperature Global Temperature Trends Mauna Loa • The Earth’s temperature increased an average of Figure 3.5 page 104 • • 0.6 °C from 1880 to 2000; but this may be a short term fluctuation since 120 years are short in comparison to the 4.5 billion history of the Earth. Doubling CO2 levels will increase temperature by 1.0-3.5 °C , smaller than Arrhenius’s prediction of 5-6 in 1896. Absorption of infrared radiation depends on molecular vibrations. Figure 3.6 Page 105 Lewis Structures and Molecular Shapes • Prediction of molecular shapes and properties • • Methane (CH4) vs. CFC-11 •Tetrahedral •4 bp & 0 lp •Non-polar CH4 •Slightly polar CFC-11 Fig. 3.8 Pg. 108 from Lewis structures. Octet Rule requires that each atom has 8 or 4 pairs of electrons; either bonding pairs (bp) and lone pairs (lp). Molecular geometry is determined by the number of lp and bp as well as the nature of electronic interaction: lp-lp>lp-bp>bp-bp (decreasing repulsion→) Figure 3.9 Page 109 •Ammonia (NH3) -Triangular pyramid •Lone pair on N pushes 3 bonding pairs, N-H, downward. Figure 3.10 Pg. 109 H-O-H angle = 104.5° Water (H2O) has 2 lp & 2 bp Shape = bent or angular CO2-Linear with double bonds O3 has resonance forms (mixed single and double bonds) Fig. 3.11 Pg. 110 Fig. 3.12 Pg. 111 Greenhouse Gases • CO2 linear • CH4 tetrahedron • H2O bent • CFCs tetrahedron • NH3 triangular pyramid • 3.9 Your Turn page 110 • 3.10 Your Turn page 111 Infrared (IR) Absorption by Molecules • Bonds absorb IR radiation that result in a change of the vibrational frequency; but IR is not energetic enough to cause bond dissociation. • The specific vibrational frequency for the absorption occurs is measured by an IR spectrometer. • The plot of radiation intensity or absorbance vs. λ is known as an IR spectrum. Figure 3.13 Page 112 Fig. 3.14 Figure 3.14 page 113: IR Spectrum of CO2 •CO2 absorbs IR photons with its energy being promoted from ground state to excited state. •Different molecular vibrational modes have different energy. Interaction between Energy and Matter Fig. 3.15 Page 113 Water vapor Spectrum Wavenumber =1/λ Spectrum of energy absorption provides information about the nature of molecular structure and is used to identify and quantify chemical compounds. (Fig. 3.16 Pg. 114) Carbon Cycle Figure 3.13 page 115 Pg. 116 CO2 output = CO2 input? Fossil Fuel contribution Carbon Cycle Pg. 117 • Sink: natural storage place in environment • • • that removes C from another part of cycle Flux: amount of C moving in the environment in 1 year Net gain of CO2 in atmosphere is about 3.1 to 3.5 Gt (gigaton) per year. Excess CO2 results in an increase of 1.5 ppm per year Mass Number • The sum of the number of protons and CO2 Emission sources in US Figure 3.17, Page 117 Avogadro’s Number • Avogadro’s Number = 6.02 x 1023 • 1 mole = 6.02 x 1023 atoms, molecules, or charged particles • Mole is a convenient unit for counting very small particles contained in gram quantities of chemical substances. the number of neutrons for a specific atom of an element is called mass number • Mass number is not the same as atomic mass! Atomic Mass of Elements • Each element has a unique atomic mass. • Atomic mass is defined as the average mass of an • • • • atom of that element as compared to an atomic mass of exactly 12 amu for a 12C atom Atomic mass can be measured by units of atomic mass unit (amu) or grams per mole. 1 amu = 1.66 x 10-24 gram Atomic mass unit (amu) is too small to measure using balances ∴Laboratory measurements of mass are reported in grams Example: Mass of an Atom • What is the mass of 1 oxygen atom expressed in grams? • Atomic mass is 15.9994 grams of oxygen per mole of oxygen atoms • Since 1 mole contains 6.02 x 1023 oxygen atoms, atomic mass divided by Avogadro Number gives the mass of one oxygen atom. Chemical Reaction & Moles • C + O2 → CO2 • The numbers of atoms and molecules involved in a reaction are proportional to the number of moles of the substances expressed in a balanced chemical equation (i.e. stoichiometric coefficients). Example Continued • 15.9994 g oxygen = 6.02 x 1023 oxygen atoms 2.66 x 10-23 g oxygen oxygen atom • Practice 3.19 Your Turn on Page 121 for the mass of a nitrogen atom and five trillion atoms. Page 122 Molar Mass • The mass of one Avogadro’s number (i.e. 1 mole) of molecular formula units of a chemical compound expressed in grams. • Molar mass is calculated by summing the atomic masses according to the molecular formula. Methane • CH4 in natural gas is an important greenhouse gas. • CH4 is produced in cattle farming, rice growing, petroleum refining, termite mounds, and landfills. • 30 times more efficient than CO2 in trapping IR • But there is less CH4 (~1.8 ppm) than CO2 (~370 ppm) in the atmosphere! Example: Molar Mass Calculation • What is the molar mass of NH3? • Find atomic mass for 1 mole of each atom in the molecule: N=14.01 g; H=1.008 g • For NH3, add the mass of nitrogen to that of hydrogen! (3 x 1.008) g + 14.01 g = 17.034 g • Molar mass of NH3 = 17.034 g per mole of NH3 • Mass of one NH3 molecule = 17.034 amu Table 3.4 on Page 125 Figure 3.19 Page 126 Nitrous oxide • N2O or “laughing gas” is used as inhaled • • • Methane in Ice Deposit from Ocean Floor Table 3.5 on Page 126 dental/medical anesthetic. Anthropogenic sources of N2O are synthesized fertilizers and burning of biomass. Agriculture exacerbates N2O problem. Also plays a role in O3 depletion, which has a cooling effect in the stratosphere Climate Modeling • Many factors are involved; examples include • • Greenhouse factor is a value that represents the relative contribution of a molecule of a substance to global warming. • solar radiation, wind patterns, cloud cover, volcanic activity, dust and soot, ocean currents, and living things. Intertwined factors make it difficult to study and the effect of CO2 independently. Computers are used to project “what if” scenarios. IPCC (Intergovernmental Panel of Climate Change) reaffirms the role of CO2 in warming. Figure 3.21 Page 129 Figure 3.22 Page 130 Table 3.7 Page 131 Fig. 3.23 Page 132 IPCC Report • CO2 and other greenhouses gases • • • contributes to an elevated global temperature. The concentration of CO2 has been increasing over the past 100 years. Increase of atmospheric CO2 is a result of human activity. Average global temperature has increased over the last 100 years. Kyoto Protocol • Global conference in 1997 to reduce emissions of greenhouse gases to “acceptable” levels • Developing countries versus industrialized countries • 2001, USA did not “sign” • But USA accounts for 25% of emissions… Fig. 3.24 Pg. 134 Blue - ↓ Red - ↑ Fig. 3.20 Fig. 3.25 Fig. 3.26 Pg. 140 Debate Topic: Stratospheric Ozone Depletion Vs. Global Warming • Based on what you have learned regarding global warming and ozone depletion, determine which is a more serious environmental threat. • Refer to Table 3.8 • Defend your position with facts