* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antidepressants in Hepatic Failure Antidepressants in Renal Failure
Hormonal contraception wikipedia , lookup
Serotonin syndrome wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
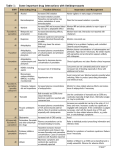
Antidepressants in Hepatic Failure Preferred Consider with Dosage Decrease Citalopram (max dose 20mg) Escitalopram Sertraline SSRIs Avoid Comments Citalopram – Levels are increased ~100% with potent Cyp2D6 inhibitors. Similar or more significant increases should be anticipated in moderate and severe HF. Fluoxetine Paroxetine Sertraline - Highly protein bound, extensively hepatically metabolized to essentially inactive metabolites. Expect significant increases in free (active) drug in severe HF. Fluoxetine - need significant dosage decrease (at least 50%), even in compensated cirrhosis. Is also potent inhibitor of CYP2D6. Venlafaxine? (↓ dose 50%) Duloxetine SNRIs Paroxetine – t ½ and AUC doubled in alcoholic cirrhosis, also potent inhibitor of CYP2D6. Venlafaxine: Studies in CYP2D6 Genetically Poor Metabolizers show decreased efficacy and increased side effects. This may be true with moderate to severe hepatic failure as well. SSRIs are likely more predictably efficacious. Duloxetine: Significant increases in AUC, even in compensated cirrhosis. Other If necessary to use, monitor serum concentration. Patients with decreased CYP2D6 (including moderate to severe hepatic failure) will have non-linear kinetics (example, a 10% increase in dose may cause a 75% increase in serum concentrations). Toxicities include delirium, seizures, and fatal arrhythmias. Mirtazepine – Approximate 33% decrease in hepatic clearance with slight increase in t1/2 in cirrhosis, likely insignificant. Amitriptyline Desipramine Nortriptyline TCAs Mirtazepine Antidepressants in Renal Failure Preferred SSRIs SNRIs Sertraline Escitalopram? Citalopram? Duloxetine* Consider with Dosage Decrease Comments Citalopram/Escitalopram – A small % (10-15%) is excreted unchanged and 10% as weakly active metabolites. Monitor for side effects in renal failure. Fluoxetine – Single dose studies show no change in PK. Many references suggest no change in dose in renal failure. However, would anticipate possible accumulation and non-linear PK with multiple doses. Paroxetine – AUC and t½ increase as renal function declines. In eCrCl <30mL/min, start at 10 mg and increase to max of 30 mg. Duloxetine – No dose adjustment needed in mild-moderate renal failure Duloxetine (↓ dose 50% in ESRD) Venlafaxine? (↓ dose 50%) Amitriptyline Desipramine Nortriptyline TCAs Other Avoid Fluoxetine? Paroxetine Mirtazepine Bupropion Hydroxy metabolites of TCAs will accumulate in renal failure. These metabolites are the most cardiotoxic form of TCAs. Mirtazepine – Clearance decreased 33% in eCrCl ~30mL/min, 50% in <10ml/min with single dose studies. Bupropion – Significant accumulation of active metabolites. SSRIs – Selective Serotonin Reuptake Inhibitors; PK – Pharmacokinetics; AUC – Area Under the Curve; t ½ - half life; eCrCl – estimated Creatinine Clearance; SNRIs – Serotonin-Norepinephrine Reuptake Inhibitors; ESRD – End-stage Renal Disease; TCAs – Tricyclic Antidepressants; HF – Hepatic Failure Author(s): Hartman A; Department: Pharmacy; Date Originated: May 2012 This information has been developed by the authors and department listed above at The Ohio State University Medical Center and is intended for use only within the institution. The information is not meant to be applied rigidly and followed in all cases. Professional judgment must remain central to the application of this information. All rights reserved, including right of reproduction, in whole or in part, in any form without permission from The Ohio State University Medical Center. Copyright: © 2012 The Ohio State University Medical Center