* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Freshman Science Exam Review
Nuclear structure wikipedia , lookup
Classical central-force problem wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Density of states wikipedia , lookup
Internal energy wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Kinetic energy wikipedia , lookup
Gibbs free energy wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Hunting oscillation wikipedia , lookup
Work (physics) wikipedia , lookup
Heat transfer physics wikipedia , lookup
Newton's laws of motion wikipedia , lookup
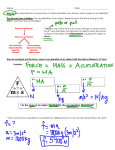
Freshman Science Exam Review Mrs. Nixon The review Sheet is for your benefit. You will be allowed to use your journal on the exam, but not your review sheet (hint: Do the review in your journal or prep journal based on review) CHAPTER 1 - nature of science Science Technology Pseudoscience Scientific method Observation Inference Manipulated (independent) Variable Responding (dependent) variable Controlled variable Constants Scientific theory Scientific law Scientific notation Significant figures Conversion figures Precision Accuracy Dimensional analysis Measurements (logical, performing, estimating) Units of o Length o Mass o Volume o Density Graphs o Slope o Direct proportion o Indirect proportion o Plot o Read and interpretxz007A Practice (pg. 31, standardized test prep) CHAPTER 2 – properties of matter Pure substance Solution Element Atom Compound Homogeneous mixture Heterogeneous mixture Suspension Colloid Physical property Physical change Viscosity Conductivity Malleability Melting point Boiling point Filtration Distillation Chemical property Chemical change Flammability Reactivity Precipitate Practice (pg. 65, standardized test prep) CHAPTER 3 – states of matter (3.1 and 3.3 only) Solid Liquid Gas Kinetic energy Phase changes Endothermic Exothermic Heat of vaporization Heat of fusion Evaporation Vapor pressure Condensation Sublimation Deposition Practice (pg.97 (1, 2, 3, 6), standardized test prep) CHAPTER 6 – chemical bonds (6.1, 6.2, 6.3) Electron dot (lewis dot) diagram Ion Anion Cation Chemical bond Ionic bond Chemical formula Covalent bond Molecule Polar covalent bond Nonpolar covalent bond Polyatomic ion Practice (pg. 189,#1-5, standardized test prep) CHAPTER 4 – atomic structure Proton Electron Neutron Atomic number Atomic mass Mass number Nucleus Isotope Energy levels Electron cloud Electron configuration Orbital Ground state Practice (pg. 65, standardized test prep) CHAPTER 7 – chemical reactions (7.1 and 7.2 only) Reactants Products Chemical equations Coefficients Subscripts Law of conservation of mass Synthesis reaction Decomposition reaction Single replacement reaction Double replacement reaction Combustion reaction Practice (pg. 225, #1-4, standardized test prep) CHAPTER 5 – periodic table Periodic table Period Group Periodic law Amu Metals Nonmetals Metalloids Transition metals Halogens Noble gases Alkali earth Alkali metals Valence electron Practice (pg. 153, standardized test prep) CHAPTER 8 – Acids and bases (8.3 and 8.4 only) Acid Base Indicator Neutralization (acid base) Reaction pH buffer electrolyte Practice (pg. 259, #3-6, standardized test prep) CHAPTER 10 – nuclear chemistry (10.1, 10.2, and 10.4 only) Radioactivity Nuclear radiation Alpha particles Beta particles Gamma rays Neutron emission Background radiation Half life Fission Fusion Practice (pg. 321, # 1-6, standardized test prep) CHAPTER 11 – motion (11.1, 11.2, and 11.3) Frame of reference Distance Displacement Speed Velocity Average speed Acceleration Distance time graphs Speed time graphs Practice (pg. 353, standardized test prep) CHAPTER 12 – forces and Motion (12.1, 12.2, 12.3) Force Newton Friction Gravity Newton’s 1st law of motion Newton’s 2nd law of motion Newton’s 3rd law of motion Mass Weight Practice (pg. 387, standardized test prep) CHAPTER 15 – energy Energy Kinetic energy Potential energy Law of conservation of energy Mechanical energy Thermal energy Nuclear energy Chemical energy Electromagnetic energy Practice (pg. 471, standardized test prep) CHAPTER 16 – thermal energy Heat Temperature Conduction Convection Radiation Insulation EQUATIONS D= m/V F = ma KE = ½ mv2 GPE = mgh S= distance/time Acceleration = speed 2 speed 1 / time 2- time 1 Sample type questions Safety 1) List 3 APPROPRIATE lab behaviors. 2) What does MSDS stand for? 3) Draw these pieces of lab equipment? 4) Test Tube 5) Test Tube Rack 6) Test Tube Holder 7) Graduated Cylinder Scientific Method 8) Are these Observations or Inferences? The sky is dark and cloudy.______ He must be a soccer player. It is going to rain._____ The Dum-Dum sucker is red. ______ He is wearing a blue shirt and yellow tie.____ The liquid is an acid._____ _____ 9) How do you write a hypothesis? 10) The Independent variable goes on the ___ axis. The Dependent variable goes on the ____ axis. 11) What is the very first step of the scientific method? 12) How do you title a graph? 13) After testing your hypothesis and making observations, may you change your hypothesis and retest it? 14) What is the last step of the scientific method? 15) Read the following experiment: You notice that your neighbor’s tomato plants are twice the size of your family’s. You want to investigate the impact on plant growth of adding various amounts of fertilizer to potted plants. You go buy the supplies—some plants and fertilizer. One plant is given fertilizer, one is not. You believe that if you start fertilizing your tomato plant, then it will grow taller. You record the initial height of each plant, and add fertilizer to one plant. Growth is recorded of each daily. You place two plants of equal size and type in a sunny windowsill and give the plants equal amounts of water – The temperature is kept the same for each plant. The plant given the fertilizer grows to 24 cm, while the other plant grows to 19 cm. As you predicted, the plant given the fertilizer grows taller than the plant without the fertilizer. What is the…. i) Control? ii) Constants? iii) Hypothesis? iv) Conclusion? v) Independent variable? vi) Dependent variable? 16) 17) 18) 19) 20) 21) 22) 23) 24) 25) 26) Metric System What is the abbreviation for decimeters? _____ Kilometer? _____ Centimeter? _____ What does “mg” stand for? ____ What does “s” stand for? _______ ms? ______ What does “mL” stand for? _______ The standard metric unit for temperature is ___________. The metric unit measured using a stopwatch is the ____________. Bob measured the length of the classroom. The units would be ________________. A graduated cylinder is used to measure __________________. _____________ is measured using a triple-beam balance. With a triple beam balance, what is the unit that goes with the measurement number? _______ There are ____ centimeters in one meter. 27) There are _____ meters in one kilometer. 28) Every measurement has a number and _______________. 29) Convert these problems into scientific notation: a) 4978270 = _____________________ b) 0.000729 = _____________________ 30) Convert these problems into standard form: a) 1.01 x 10-6 b) 31.00 x 104 = _____________________ = _____________________ 31) How many significant figures are in the following numbers? a) 7080 = ____ 4.070 = ____ 0.0076 = ____ 80.000 = ____ Graphing Graph A 32) What type of graph is A? 33) What type of graph is B? Matter 34) Solid, liquid, gas, or plasma? a) A substance that has a definite volume, but changing (indefinite) shape: b) A substance that does NOT have a definite volume nor a definite shape: c) A substance that has a definite volume and a definite shape Graph B 35) Fill in the diagram with the processes. GAS . SOLID LIQUID 36) 37) 38) This phase change diagram is for what substance? ______ What is happening at point 3? _______________ What is happening at point 4? _________________ 39) According to the diagram below, which beaker contains the densest liquid? Substance A B C Melting Point - 67 C 5C 189 C 40) What substance(s) is a gas at 90 C? __________ 41) What substance(s) is a liquid at 186 C? ________ Boiling Point -10 C 234 C 191 C 42) Physical Change or Chemical Change? i) Burning wood ____________ ii) Iron rusting _______________ iii) Copper turning green _______________ iv) Ice melting ________________ v) Mashing bananas _______________ 43) Two identical graduated cylinders containing different liquids are placed on identical balances. What Conclusion about density can you make based only on what you can observe from the diagram below? A 44) B At 25 ºC, water has a density of 1.0 g/mL and vegetable oil has a density of 0.90 g/mL. Substance Z has a density of 0.95 g/mL. Choose which letter best represents Substance Z when it is place in water and oil. i. A ii. B iii 45) 46) C A buckeye has a mass of 17.89 g. When placed in a graduated cylinder with 20.0 mL of water, the water rose to 50.7 mL. What is the density of the buckeye? A pure diamond has a density of 4.52 g/cm 3. Its mass is 7.81g. What is the volume of the diamond? 47) Matter is defined as anything that…____________ Use these words below. Chemical . Physical Elements Heterogeneous . Suspension Colloid 48) Pure substances are either _________________ or compounds. 49) In a(an) _________ mixture, the parts of a mixture are noticeably different from one another. 50) If the particles in a mixture, such as fog, scatter light, the mixture is a(an) _____. 51) A(An) __________ change has taken place when the material has changed shape or size, but the composition of the material remains the same. 52) When the substances in a sample of matter are changing into different or new substances, _____ changes may be observed. 53) When the parts of a mixture settle into different layers, you have created a(an) ___________. 54) Is this a compound? Cobalt, Co Carbon Dioxide, CO2 sodium, Na iodine, I 55) Is this a pure substance? Elements Mixtures Compounds 56) Is this a mixture? Carbon Dioxide (CO2) Iron (Fe) Soil 57) A mixture that appears to contain only one substance (the same throughout) is a(an) 58) Is this a heterogeneous mixture? water in a swimming pool sugar water a jar of mixed nuts stainless steel 59) Is this a physical or chemical change? water boils at 100 Celsius an aspirin tablet is crushed balls of wax form when melted wax is poured into ice water a gas forms when vinegar and baking soda are mixed Atoms & The Periodic Table 60) Which scientist discovered that atoms consist of subatomic, charged particles? _______ 61) Who discovered the nucleus?_______ 62) Who thought atoms of a solid were rough and atoms of a liquid were smooth? 63) Who thought of an atom as a Plum Pudding Model?_________ 64) The subatomic particle found in the nucleus and has no charge is… 65) In order to find the atomic mass of an element, one must add together the number of which 2 subatomic particles? 66) Elements on the periodic table are arranged according to their ___________ ____________… 67) 68) 69) 70) 71) Which element will you find in the 3rd row, 2nd column? ____________ How many electrons can the 3rd energy level hold? ______ The number of energy levels an atom has corresponds to the number of…__________ Francium and Cesium are both elements in which family of elements? ___________ How are elements arranged in the Periodic Table? By increasing __________. 72) Where is most of the mass of an atom located? _________ 73) What column number are the Halogens? __________ 74) How many families (certain sections we studied) are there in the Periodic Table? 75) How many rows are there in the Periodic Table? __________ 76) What element will you find in row 2, column 14? __________ __________ 77) Which Noble gas is in row 5 of the periodic table? __________ 78) 79) 80) 81) Elements in the middle of the table are the __________. In a NEUTRAL atom, calcium has: How many p? ____n? ____e? ______ In a NEUTRAL atom, tin (Sn) has: How many p? ____n? ____e? ______ Draw the Bohr model for each of the following…Draw the Lewis Dot structure, too! i) Fluorine Chlorine ii) Potassium Magnesium 82) Describe the characteristics of Noble gases. 83) What are isotopes? 84) What is the same between oxygen-17 and oxygen-18? Different? 85) The charge of an electron is _____ 86) What are valence electrons? 87) Where would you likely find elements on the periodic table that form negative ions? 88) How many electrons does an ion of fluorine with a charge of -1 contain? _____ 89) What family are the following elements in? i) Chlorine ________ ii) Calcium ________ iii) Aluminum ________ 90) How many valence electrons do the following elements contain? i) Beryllium ________ ii) Oxygen ________ iii) Carbon _________ 91) How many energy levels do the following elements contain? i) Lithium ii) Potassium _______ iii) Sulfur _________ _________ 92) Ionic or Covalent? i) CH4 ii) NaCl ________ _________ iii) BaSO4 ___________ iv) electrons are transferred _______ v) electrons are shared ________ 93) Balance these equations. i) ___ Cl2 + ___ NaBr ___ NaCl + ___Br2 ii) ___Al2O3 ___Al + ___O2 iii) __H2 + ___O2 ___H2O 94) An endothermic reaction is one that ______ energy, where an exothermic reaction _______ energy. 95) WHY must all chemical reactions be balanced? 96) Match these to their points. Neutral Strong acids 1 2 3 4 5 6 Strong bases 7 8 9 Weak acids 10 11 12 Weak bases 13 14 97) Acids taste…_________ 98) All acids contain which ion? ______________ 99) Bases taste…____________ 100) All bases contain which ion? _______________ 101) You find an unknown liquid and want to determine if the substance is an acid or a base. You test the liquid with blue litmus paper. It turns red. You can conclude the liquid is a(n)…_________________ 102) Bases feel…______________ 103) Arrange the following substances in order of increasing acidity: vinegar (pH=2.8), gastric juices from inside your stomach (pH = 2.0), a soft drink (pH = 3.4) and baking soda (pH = 9). 104) The Lewis Dot structure for Aluminum, has ____ electrons around the symbol Al. 105) The chemical formula for dihydrogen monoxide is… _______ 106) The chemical formula for Magnesium Fluoride is… _________ Nuclear Energy 107) The process of nuclear change in an atom of radioactive material is called…_______ 108) The process of the production of lighter nuclei from heavier nuclei is called…_______ 109) Fusion occurs when nuclei…_________ 110) __________ can be stopped by a piece of paper. Motion and Forces 111) A push, a pull or a lift on an object. ________ 112) The tendency of motion not to change______ 113) Represents speed in a particular direction of an object. 114) 115) 116) 117) 118) 119) 120) If an object is at rest, then the forces acting on the object must be An object that travels equal distances in equal time must be traveling. Assumed to be stationary so the motion of objects may be predicted. Pull of gravity on an object. If an Object is moving, the forces must be… SI unit for force.______ Which Law of Newton? i) The penny continuing to move when the cart stops ii) A ball with more mass will have a greater force iii) A basketball shot bouncing off of the backboard iv) A penny at rest falls into the cup when the card it rests on is removed v) A empty shopping cart accelerates at a faster rate than a full shopping cart vi) When one falling domino bumps into another and that one bumps into the next, the chain reaction occurs because of ____. vii) A marble will continue to roll in a straight line at constant speed unless the force of friction causes it to stop according to ____. viii) A 5 kg object having an acceleration of 10 m/s2 has a force of 50 N because of ____(F=ma). 121) What three ways can an object accelerate? 122) A runner travels 800 meters in 80 seconds, what is the runner’s average speed? 123) An object accelerates when it falls to the ground because: 124) The amount of force acting on a moving object can be measured by determining the object’s mass & _______________. 125) If in a tug-of-war game, the red team is pulling with a force of 100 N & the blue team is pulling in the opposite direction with a force of 60 N, then who will win? 126) Based on Newton’s laws of motion, a hockey puck sliding across the ice slows down because 127) How much force is needed to accelerate a 78 kg skier 8 m/s/s? 128) 129) What is velocity? What is the reaction force when you place a cup on a table? 130) The following picture shows the motion of a snowboarder from point 1 to point 5. 131) Which graph best represents the acceleration of the snowboarder as he moves from point 2 to point 3? 132) 133) 134) 135) 136) Which graph(s) depict an object that is not moving? Which graph(s) depict an object moving at a constant speed? Which graph(s) depict an object that is accelerating? What does the slope indicate in graphs A and B? What does the slope indicate in graphs C and D? Energy 137) At what time does the box have the greatest kinetic energy (which letter)? ________ 138) The weight of the box used in the experiment in 10 Newtons (N) as illustrated in the figure. The weight of the box is a measure of the ___________ 139) Where is the potential energy of the box greatest? ________ 140) The total energy of the box is… __________. 141) The picture below shows the four major forces acting on an airplane in flight. 142) i) What causes the force indicated by the X? 143) A student plans to collect data needed to calculate the kinetic energy of a thrown baseball. She plans to measure the distance from pitcher to catcher, the time it takes for the baseball to arrive in the catcher’s glove, the mass of the baseball, and the circumference of the baseball.Which of these measurements is not needed to calculate the kinetic energy? Explain why. 144) 145) 146) Radiant to _________________ A piano: Motion to ________________ A battery: Chemical to ________________ 147) What are the forms of potential energy? 148) Friction coverts some kinetic energy into ______________________ energy. 149) A 5-kilogram cat is resting on top of a bookshelf that is 2 meters high. What is the cat’s gravitational potential energy relative to the floor if the acceleration due to gravity is 9.8 m/s2? 150) A small 20-kilogram canoe is floating downriver at a speed of 2 m/s. What is the canoe’s kinetic energy? 151) 152) The total energy of an object is equal to its __________…. What will have a greater effect on the kinetic energy of an object? Mass or velocity? B ecause? 153) What is the curve of the liquid in a graduated cylinder called? IN ADDITION…Short Answer/Extended Reponses Questions 154) Compare and contrast fission and fusion? 155) What is the difference between distance and displacement? 156) How can you tell if a chemical change occurred? 157) A ship engine is capable of an acceleration speed equal to -0.357 m/s2. Suppose the ship approaches the dock at a velocity of 16.98 m/s. How much time does the ship need to stop? 158) Find the average acceleration of a northbound subway train that slows down from 12 m/s to 9.6 m/s in 0.80 s. 159) If the average speed of a car is 45 km/hr, how far can it travel in 40 min? 160) Falling objects drop with an average acceleration of 9.8 m/s 2. If an object falls from a tall building, how long will it take before it reaches a speed of 49 m/s? 161) What is the Law of Conservation of Mass? What is Law of Conservation of Energy? 162) What are Newton’s 3 laws? 163) Draw Bohr model and Lewis dot structure of Lithium. 164) Show ionic bonding between NaF. Show covalent bonding between CH 4.