* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Drug discovery wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
History of chemistry wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical bond wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Computational chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Mass spectrometry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Thermometric titration wikipedia , lookup
History of molecular theory wikipedia , lookup
Implicit solvation wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Geometrical frustration wikipedia , lookup
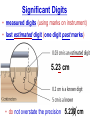
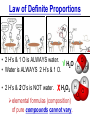
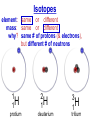
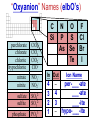
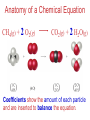
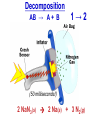
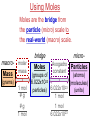
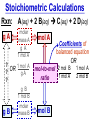
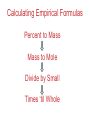
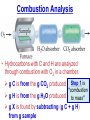
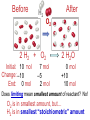
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Unit 3 (Chp 1,2,3): Matter, Measurement, & Stoichiometry John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall Inc. Chemistry: The study of matter and the changes it undergoes. Ni nickel solid metal + H2 HCl hydrochloric acid aqueous solution + NiCl2 hydrogen nickel(II) chloride gas solid crystals Quantitative or Qualitative Matter Atom: simplest particle with properties of element Element: same type of atom (1 or more) HH H2 O O O2 C C Na C C C Compound: different atoms bonded molecule H2O CO2 NaCl chromatography distillation (boiling) Matter separate physically Mixture differences or unevenly mixed filtering Heterogeneous Mixture (suspensions/colloids) Physical changes uniform or evenly mixed Homogeneous Mixture (solutions) cannot separate physically Pure Substance separate chemically cannot separate Chemical changes Compounds Elements salt, baking soda, oxygen, iron, water, sugar hydrogen, gold NaCl NaHCO3 O2 Fe H2O C12H22O11 H2 Au Physical Separation: Filtration: Separates heterogeneous mixtures (solids from liquids). Physical Separation: Distillation: Separates solution by boiling point differences. Physical Separation: Chromatography: Separates solution by differences in solubility (attractions). Metric Prefixes Prefix Symbol Multiplier Examples: 1,000,000,000 B 1,000,000 J 1,000 g BASE UNIT: 1m 1L 1g 0.01 m 0.001 L (light wavelength) (atoms) (nuclei) 0.000 001 g 0.000 000 001 m Precision in Measurements Measuring devices have different uses and different degrees of precision. (uncertainty) % Error = |Experimental – Accepted| x100 Accepted Significant Digits • measured digits (using marks on instrument) • last estimated digit (one digit past marks) 5.23 cm • do not overstate the precision 5.230 cm Significant Zeroes 0.0003700400 grams 0’s 1. All nonzero digits are significant. 2. Captive Zeroes between two significant figures are significant. 3. Leading Zeroes at the beginning of a number are never significant. 4. Trailing Zeroes: Sig, if there’s a decimal point. NOT, if there is no decimal point. Scientific Notation Power of 10 is the number of places the decimal has been moved. Examples: 42000 = 4.2 x 104 0.0508 = 5.08 x 10–2 positive power: move decimal right to obtain the original # in standard notation. negative power: move decimal left to obtain the original # in standard notation. Scientific Notation 1. Convert the numbers to scientific notation. 2.45 x 104 (i) 24500 9.85 x 10–4 (ii) 0.000985 (iii) 12002 1.2002 x 104 2. Convert to standard notation. 420,000 (i) 4.2 x 105 0.000215 (ii) 2.15 x 10-4 0.003 (iii) 3 x 10-3 Sigs Digs in Operations + or – round answers to keep the fewest decimal places 3.48 + 2.2 = 5.68 x or ÷ 5.7 round answers to keep the fewest significant digits 6.40 x 2.0 = 12.8 13 Sig Digs Practice WS 1s 1. How many sig digs are in each number? 4 (i) 250.0 (ii) 4.7 x 10–5 2 (iii) 34000000 2 4 (iv) 0.03400 2. Round the answer to the correct sig digs. (i) 34.5 x 23.46 809 (ii) 123/3 40 (iii) 23.888897 + 11.2 35.1 (iv) 2.50 x 2.0 – 3 2 WARM UP (for QUIZ!!!) • Review WS 1s #1, 3, 10 • Complete WS 1a #1, 2, 8, 9, 10 Law of Definite Proportions • 2 H’s & 1 O is ALWAYS water. • Water is ALWAYS 2 H’s & 1 O. • 2 H’s & 2 O’s is NOT water. √ H 2O X H2O2 elemental formulas (composition) of pure compounds cannot vary. O H H H H O O Law of Conservation of Mass The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the process took place. __H 2 2 + __O2 2 2O __H Balancing Equations!!! Symbols of Elements Mass Number = p’s + n’s 12 C 6 Element Symbol Atomic Number (Z) = p’s All atoms of the same element have the same number of protons (same Z), but… can have different mass numbers. HOW? Isotopes element: same or different mass: same or different why? same # of protons (& electrons), but different # of neutrons 1 H 1 protium 2 H 1 deuterium 3 H 1 tritium Average Atomic Mass • average atomic mass: calculated as a weighted average of isotopes by their relative abundances. • lithium-6 (6.015 amu), which has a relative abundance of 7.50%, and • lithium-7 (7.016 amu), which has a relative abundance of 92.5%. (6.015)(0.0750) + (7.016)(0.925) = 6.94 amu Avg. Mass = (Mass1)(%) + (Mass2)(%) … Mass Spectrometry atomized, ionized magnetic field element sample isotopes separated WS Atomic by Structure difference Cl (avg at. Mass) = in mass (35)(~0.75) + (37)(~0.25) = ? ~75% ~25% Molecular (Covalent) Compounds Covalent compounds contain nonmetals that “share” electrons to form molecules. (molecular compounds) Diatomic Molecules “H-air-ogens” 7 These seven elements occur naturally as molecules containing two atoms. Binary Molecular Compounds • list less electronegative atom first. (left to right on PT) • use prefix for the number of atoms of each element. • change ending to –ide. CO2: carbon dioxide CCl4: carbon tetrachloride pentoxide N2O5: dinitrogen ________________ CuSO4∙5H2O (ionic & covalent) copper(II) sulfate pentahydrate Cations metals lose e’s (+) charge (metal) ion Anions Ions nonmetals gain e’s (–) charge (nonmetal)ide Ionic Bonds Attraction between +/– ions formed by metals & nonmetals transferring e–’s. Formulas of Ionic Compounds • Compounds are electrically neutral, so the formulas can be determined by: – Crisscross the charges as subscripts (then erase) – If needed, reduce to lowest whole number ratio. Pb4+ O2– Pb2O4 PbO2 Naming Ionic Compounds 1) Cation: Write metal name (ammonium NH4+) For transition metals with multiple charges, write charge as Roman numeral in parentheses. Iron(II) chloride, FeCl2 Iron(III) chloride, FeCl3 2) Anion: Write nonmetal name with –ide OR the polyatomic anion name. (–ate, –ite) Iron(II) sulfide, FeS Magnesium sulfate, MgSO4 Common Polyatomic Ions * these 12 will be on Quiz 1 - all 20 Polyatomic Ions will be on Quiz 2 WS 2d Name Symbol Charge *ammonium NH4+ 1+ *acetate C2H3O2– 1– – (ethanoate) (CH3COO ) *hydroxide OH– 1– *perchlorate ClO4– 1– *chlorate ClO3– 1– chlorite ClO2– 1– hypochlorite ClO– 1– bromate BrO3– 1– iodate IO3– 1– *nitrate NO3– 1– nitrite NO2– 1– cyanide CN– 1– *permanganate MnO4– 1– *bicarbonate 1– HCO3– (hydrogen carbonate) *carbonate CO32– 2– *sulfate SO42– 2– sulfite SO32– 2– *chromate CrO42– 2– dichromate Cr2O72– 2– *phosphate PO43– 3– “Oxyanion” Names (elbO’s) C Si N O P S As Se Te perchlorate chlorate chlorite hypochlorite ClO4– ClO3– ClO2– ClO– nitrate nitrite NO3– NO2– In Out 4 – sulfate sulfite SO42– SO32– phosphate PO43– 3 2 1 4 3 – F Cl Br I Ion Name per-___-ate ___-ate ___-ite hypo-___-ite Naming Acids Ion add H+ Acid In Out Ion Name Acid Name 4 – per-___-ate per-___-ic acid 3 4 ___-ate ___-ic acid 2 3 ___-ite ___-ous acid 1 – hypo-___-ite hypo-___-ous acid perchlorate Name Acids chlorate from these chlorite oxyanions: hypochlorite WS 2e – ClO4 ClO3– ClO2– ClO– nitrate NO3– nitrite NO2– sulfate SO42– sulfite SO32– Anatomy of a Chemical Equation CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) Anatomy of a Chemical Equation CH4(g) + 2 O2(g) Reactants appear on the left side of the equation. CO2(g) + 2 H2O(g) Anatomy of a Chemical Equation CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) Products appear on the right side of the equation. Anatomy of a Chemical Equation CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) States (s, l, g, aq) written in parentheses next to each compound Anatomy of a Chemical Equation CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) Subscripts show how many atoms of each element Anatomy of a Chemical Equation CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) Coefficients show the amount of each particle and are inserted to balance the equation. Reaction Types Combination A + B → AB Demo: MgO 2 Mg(s) + 2→1 O2(g) 2 MgO(s) Decomposition AB → A + B 1→2 (50 milliseconds!) 2 NaN3(s) 2 Na(s) + 3 N2(g) Replacement Reactions (or “Displacement”) Single Replacement AB + C → A + CB (aq) + (s) (s) + video (aq) Double Replacement AB + CD → AD + CB Pb(NO3)2(aq) + KI(aq) PbI2(s) + KNO3(aq) Demo: Combustion CxHy + _O2 _CO2 + _H2O • Often involve hydrocarbons reacting with oxygen in the air WS 4a CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) Formula Weights Formula Weight (FW) Molecular Weight (MW) • Sum of the atomic weights for the atoms in a chemical formula • Formula Weight of calcium chloride, CaCl2, is… • Sum of the atomic weights for the atoms in a molecule or compound • Molecular Weight of ethane, C2H6, is… Ca: 1(40.08 amu) + Cl: 2(35.45 amu) 110.98 amu C: 2(12.01 amu) + H: 6(1.008 amu) 30.07 amu Percent Composition (# of atoms)(AW) x 100 % element = (FW) One can find the percent by mass of a compound of each element in the compound by using this equation. Percent Composition So the percentage of carbon in ethane (C2H6) is… %C = = (2)(12.01) (30.07) 24.02 x 100 30.07 = 79.88% C Moles Avogadro Constant • One mole of particles contains the Avogadro constant of those particles 6.022 x 1023 Mole Relationships • One mole of atoms, ions, or molecules contains the Avogadro constant of those particles 6.022 x 1023 In 1 mol Na2CO3 , how many… • Na atoms? • C atoms? • O atoms? • How many donuts in 1 mol of donuts? • How many boogers in 1 mol of boogers? Which has more atoms, 1 mol CH3 or 1 mol NH3 ? How about CH3CH2OH or H2SO4 ? Molar Mass • the mass of 1 mol of a substance (g/mol) – molar mass (in g/mol) of an element is the atomic mass (in amu) on the periodic table – formula weight (amu) of a compound same number as the molar mass (g/mol) of 1 mole of particles of that compound Using Moles Moles are the bridge from the particle (micro) scale to the real-world (macro) scale. bridge macro- Mass molar mass Moles microAvogadro constant Particles 1 mol #g (groups of (atoms) 6.022x1023 (molecules) 23 particles) 6.022x10 (units) 1 mol #g 1 mol 1 mol 6.022x1023 (grams) Using Moles 1.What is the mass of 1 mole of copper(II) bromide, CuBr2?(63.55) + 2(79.90) = 223.35 g = 6.022 x 1023 particles 2.How many moles are there in 112 g of copper(II) bromide, CuBr2? 1 mol CuBr2 = 0.501 mol 112 g CuBr2 x 223.35 g CuBr2 CuBr2 3.How many particles present in each of the questions #1 & #2 above? 6.022 x 1023 particles = 3.02 x 1023 0.501 mol x 1 mol particles Stoichiometry: calculations of quantities in chemical rxns –how much reactant is consumed or –how much product is formed •Balanced chemical equations show the amount of: atoms, molecules, moles, and mass Most important are the ratios of reactants and products in moles, or… mol-to-mol ratios Stoichiometric Calculations Rxn: gA A(aq) + 2 B(aq) C(aq) + 2 D(aq) molar mass A g A 1 mol A ? 1 mol A OR ? gA ? g B 1 mol B molar gB mass B mol A Coefficients of balanced equation OR mol-to-mol 2 mol B 1 mol A ratio mol B 1 mol A 2 mol B Stoichiometric problems have 1-3 Steps: (usually) 1) Convert grams to moles (if necessary) using the molar mass (from PT) 2) Convert moles (given) to moles (wanted) using the mol ratio (from coefficients) 3) Convert moles to grams (if necessary) using the molar mass (from PT) grams A x 1 mol A x _ mol B x grams A mol A . grams B = 1 mol B 1) molar mass 2) mole ratio 3) molar mass Stoichiometric Calculations Example : g of A g of B HW p. 114 #58 Solid magnesium is added to an aqueous solution of hydrochloric acid. What mass of H2 gas will be produced from completely reacting 18.0 g of HCl with magnesium metal? Mg(s) + 2 HCl(aq) MgCl2(aq) + H2(g) mole ratio B/A molar mass B 2.016 g H2 1 mol HCl x 1 mol H2 x 18.0 g HCl x 36.46 g HCl 2 mol HCl 1 mol H2 g of A molar mass A = ____ g Hg2 H2 0.498 Finding Empirical Formulas Types of Formulas • Empirical formulas: the lowest ratio of atoms of CH 3 each element in a compound. C2H4O • Molecular formulas: C2H6 C6H12O3 the total number of atoms of each element in a compound. molecular mass = emp. form. empirical mass multiple Calculating Empirical Formulas Steps (rhyme) from Mass % Composition Percent to Mass assume 100 g Mass to Mole MM from PT ÷ moles by smallest to CH 4 Divide by Small get mole ratio of atoms x (if necessary) to get Times ‘til Whole whole numbers of atoms 75 % C 75 g C 6.2 mol C 1C 25 % H 25 g H 24.8 mol H 4H 1) Percent to Mass 3) Divide by Small 2) Mass to Mole 4) Times ’til Whole Butane is 17.34% H and 82.66% C by mass. Determine its empirical formula. 82.66 g C x 1 mol C = 6.883 mol C = 1 1 C x 2 12.01 g C 6.883 mol =2C 17.34 g H x 1 mol H = 17.20 mol H 1.008 g H 6.883 mol = 2.499 2.5 H C2H5 x 2 =5H If molecular mass is 58 g∙mol–1, what is the Molecular Formula? HW p. 113 #43a, 48 molecular mass empirical mass 58 =2 29.06 2 (C2H5) = C4H10 Calculating Empirical Formulas Percent to Mass Mass to Mole Divide by Small Times ‘til Whole Combustion Analysis • Hydrocarbons with C and H are analyzed through combustion with O2 in a chamber. g C is from the g CO2 produced Step 1 is “combustion g H is from the g H2O produced to mass” g X is found by subtracting (g C + g H) from g sample Combustion Analysis Example 1 When 4-ketopentenoic acid is analyzed by combustion, a 0.3000 g sample produces 0.579 g of CO2 and 0.142 g of H2O. The acid contains only C, H, and O. What is the empirical formula of the acid? 1 mol CO2 1 mol C 12.01 g C 0.579 g CO2 x x x 44.01 g CO2 1 mol CO2 1 mol C ?gC = 0.158 g C Step 1: “combustion to mass” 1 mol H2O x 2 mol H x1.008 g H 0.142 g H2O x 18.02 g H2O 1 mol H2O 1 mol H ?gH = 0.0159 g H 0.3000 g sample – (0.158 g C) – (0.0159 g H) = ?gO = 0.126 g O 1 mol C 0.158 g C x = 0.0132 mol C = 1.67 C 12.01 g C 0.00788 mol x3=5C 1 mol H 0.0159 g H x = 0.0158 mol H = 2 H 1.008 g H 0.00788 mol x3=6H 1 mol O 0.126 g O x = 0.00788 mol O = 1 O 16.00 g O 0.00788 mol x3=3O C5H6O3 Combustion Analysis Example 2 A sample of a chlorohydrocarbon with a mass of 4.599 g, containing C, H and Cl, was combusted in excess oxygen to yield 6.274 g of CO2 and 3.212 g of H2O. Calculate the empirical formula of the compound. If the compound has a MW of 193 g∙mol–1, what is the molecular formula? 1 mol CO2 1 mol C 12.01 g C 6.274 g CO2 x x x 44.01 g CO2 1 mol CO2 1 mol C ?gC = 1.712 g C Step 1: “combustion to mass” 1 mol H2O x 2 mol H x1.008 g H 3.212 g H2O x 18.02 g H2O 1 mol H2O 1 mol H ?gH = 0.3593 g H 4.599 g sample – (1.712 g C) – (0.3593 g H) = ? g Cl = 2.528 g Cl 1 mol C 1.712 g C x = 0.1425 mol C = 2 C 12.01 g C 0.07131 mol 1 mol H 0.3593 g H x = 0.3564 mol H = 5 H 1.008 g H 0.07131 mol 1 mol Cl 0.07131 mol Cl 2.528 g Cl x = 1 Cl = 35.45 g Cl 0.07131 mol C2H5Cl If the compound has a MW of 193 g∙mol–1, what MW 193 =3 is the molecular formula? EW 64.51 HW p. 114 #52b C6H15Cl3 How Many Cookies Can I Make? • Which ingredient will run out first? • If out of sugar, you should stop making cookies. • Sugar is the limiting ingredient, because it will limit the amount of cookies you can make. Before H2 After O2 Which is limiting? 2 H2 + O2 Initial: 10 ? mol Change: –10 End: 0 mol limiting ?7 mol –5 2 mol excess 2 H2O ?0 mol +10 10 mol Before H2 After O2 2 H2 + O2 Initial: 10 ? mol Change: –10 End: 0 mol ?7 mol –5 2 mol 2 H2O ?0 mol +10 10 mol Does limiting mean smallest amount of reactant? No! O2 is in smallest amount, but… H2 is in smallest “stoichiometric” amount Limiting Reactant • convert reactant A to reactant B to compare • If available < needed (limiting) • If available > needed (excess) Solid aluminum metal is reacted with aqueous copper(II) chloride in solution 2 Al + 3 CuCl2 2 AlCl3 + 3 Cu 54.0 g Al 4.50 mol CuCl2 (Which is limiting?) 54.0 g Al x 1 mol Al x 3 mol CuCl2 = 3.00 mol CuCl 2 26.98 g Al 2 mol Al (4.50 mol CuCl2) available > needed (3.00 mol CuCl2) CuCl2 is excess Al is limiting Theoretical Yield theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry – limited by LR (use LR only to calculate) limiting 54.0 g Al x 1 mol Al x 3 mol Cu x 63.55 g Cu = 191 g Cu 26.98 g Al 2 mol Al 1 mol Cu produced • different from actual yield (or experimental), amount recovered in the experiment HW p. 115 #72 Percent Yield A comparison of the amount actually obtained to the amount it was possible to make Actual %Yield = x 100 Theoretical (calculate using the LR only) NOT % Error: % Error = |Accepted – Experimental| x100 Accepted Percent Yield Aluminum will react with oxygen gas according to the equation below 4 Al + 3 O2 2 Al2O3 • In one such reaction, 23.4 g of Al are allowed to burn in excess oxygen. 39.3 g of aluminum oxide are formed. What is the percentage yield? Percent Yield 4 Al + 3 O2 HW p. 116 #79, 77 2 Al2O3 1mol Al 2 mol Al2O3 101.96 g Al2O3 23.4 g Al x x x 26.98 g Al 4 mol Al 1 mol Al2O3 = 44.2 g Al2O3 39.3 g of aluminum oxide are formed. What is the percentage yield? 39.3 g %Yield = 44.2 g x 100 88.9 %