* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Theoretical and experimental IR line shape (from B.M. Auer and J.L.
Survey
Document related concepts
Transcript
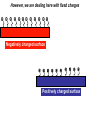
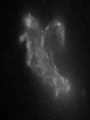
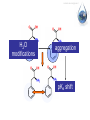
Antonella De Ninno Centro Ricerche ENEA Frascati Roma (Italy) [email protected] Water [email protected] • Gases are fully non coherent systems • Liquids are systems where electron clouds are coherent • Solids are systems where nuclei, too, are coherent • Liquid water is peculiar, since the coherent oscillation connects two electronic configurations that have extreme features: 1) The ground configuration where all electrons are tightly bound (the ionization potential is 12.60 eV, corresponding to soft X-rays and to an excitation temperature of 145.000 °C !) 2) The excited configuration has an energy E=12.06 eV, only 0.54 eV below the ionization threshold. So for each molecule there is an almost free electron! [email protected] The QED reveals us the dynamical origin of these clusters [email protected] In liquid water two phases exist. The interplay between the electrodynamic attraction and thermal disruption produces a continuous crossover of molecules between the two regimes. The QED theory foresees a dynamical distribution between the two phases Fc, Fnc of coherent and noncoherent molecules depending on the temperature: Fc (T ) Fnc (T ) 1 [email protected] How can we observe experimentally the two phases in liquid water at room temperature and pressure ? Measuring the energy differences between the two populations via FT-IR spectroscopy we can measure the energy difference between the “more correlated” and the “less correlated” kind of molecules and compare the result with the amount calculated by QED IR spectrum of liquid water 0 . 9 0 . 8 [email protected] OH stretching vibration 0 A b . 6 0 . 4 0 . 2 Bending mode of the isolated molecule s 0 - 0 . 1 4 0 0 0 3 W 0 0 a 0 v 2 e n u m b e r [ c 0 m 0 - 1 0 1 ] 0 0 0 [email protected] Experimental spectrum of water T=25°C 7 6 Molecules having a strong correlation with the environment coherent intermediate 4 Abs Monomers and/or 2 dimers non-coherent 0 4000 3500 W av enumber[cm-1] ENERGY 3000 2800 [email protected] Comparison of the gas, liquid and solid spectra of the same amount of water. From Martin Chaplin: Water Structure and Science web page http://www.lsbu.ac.uk/water/vibrat.html [email protected] Such a system will also exhibit a Van't 1. Hoff behaviour: we can observe experimentally that our system (liquid water) exhibits an equilibrium point upon changing the temperature, in fact exists a point in the IR spectrum where the absorption is always the same 2. this suggests the existence of an equilibrium between two components 3. we know from thermodynamics that at equilibrium the variation of the Gibbs’energy, i.e., the maximum amount of useful work from a reaction is equal to 0 G G0 RT ln K eq G 0 RT ln K eq Equilibrium constant can be used to evaluate thermodynamic parameters H 0 T S RT ln K eq [email protected] Which components are the at equilibrium ? 7 T1=30 °C 6 T2=40°C Monomers and/or dimers + intermediate non-coherent T3=60°C 4 A b Molecules having a strong correlation with the environment coherent s 2 0 4 0 0 0 W a 3 5 0 0 v e n u 3 m b e r [ c ENERGY m - 1 ] 0 0 0 2 8 0 0 [email protected] A plot of Keq vs. 1/T should be a straight line with H 0 intercept 0,3 RT S0 I1 E ln( ) c I2 KT 0,2 R Ln(I1/I2) slope 0,4 0,1 0 -0,1 -0,2 -0,3 2,9E-03 3,0E-03 3,1E-03 3,2E-03 3,3E-03 3,4E-03 1/T (K-1) Here the equilibrium constant is the ratio between the peak of the coherent and non-coherent + intermediate populations [email protected] Van’t Hoff plot E 0.127 0.028eV E 0.17 0.05eV Experimental (T=300K) Calculated (T=0) N.B. At T≠ 0 actually, the boundaries are not sharp because of the thermal collisions and the energy gap is decreased. [email protected] In such a picture, even the so called intermediate population could find a rationale: the measured spectrum emerges from a dipole-dipole transition between two specific quantum states the intermediate peak is naturally assigned to the transitions where the initial state is in the coherent fraction and the final state is in the noncoherent fraction and vice versa. (The average life time of the coherent state is ~ 4·10-15 sec which is about 2 times the vibration transition time scale) [email protected] [email protected] [email protected] 1.Is the dynamical distribution of the two phases only function of the temperature? 2.Is it affected by the interaction with the environment? 3.Can one phase be selectively stabilized? [email protected] Is the dynamical distribution of the two phases only function of the temperature? NaCl solution 1,5 Coherent 1,4 Non-coherent No, it also depends on the concentration of solutes 1,3 1,2 A.U. 1,1 1 0,9 0,8 0,7 0,6 0,5 -1 0 1 2 3 Mol 4 5 6 Do the interaction with the environment affect the distribution of the two phases ? [email protected] Yes, the quality of the surface modifies the percentage of the coherent phase Water near hydrophilic surfaces Coherent bulk water Coherent EZ water Intermediate bulk Intermediate EZ Non-coherent bulk Non-coherent EZ Peak position 3205 3292 3361 3494 3526 3610 Area % 58 76 37 4 5 20 t=1/Egap increased Egap decreased Negatively charged surface Water is a heterogeneous (at least a two-phase) system in which charge separation occurs between two phases : low entropy (organized) interfacial and less organized “bulk”. EZ (interfacial) ─ Up to 150 mv Bulk + EZ-water may be charged negatively or positively depending on the charge of the surface forming it Zheng JM, Wexler A, Pollack GH.. J Colloid Interface Sci. 2009 However, we are dealing here with fixed charges Ө Ө Ө Ө Ө Ө Ө Ө Ө Ө Ө Ө Negatively charged surface Positively charged surface Like charges repel each other, but as they are covalently fixed to a matrix, they all cannot but vibrate Their collective vibration could become coherent due to the principle of minimization of energy Interactions with the environment, in this case the interaction with the surface just acts like external trigger. Water appears to contain in itself the informations. This may explain why the biologic message is NOT deterministic. Can one phase be selectively stabilized? [email protected] [email protected] Light Scattering on water Light scattering gives information about the presence of large size aggregates into the liquid provided that a certain number of hypothesis in support of the Mie scattering theory are verified [email protected] [email protected] Can one phase be selectively stabilized? 3-5 drops of the liquid have been evaporated, at room temperature and pressure, on mica substrates forming solid deposits. Atomic Force Microscopy images of these deposits were taken in non-contact mode Yes, a long lasting change in the structure of liquid water can be induced by the iterative contact with Nafion membranes (not only) Residues from five drops of a sample of INW. The bright-to-dark colour coding corresponds to the height of the clusters, ranging from 0.040μm (the control - right) to 0.403μm (the sample – left). The size of the picture is 10 μm×10. [email protected] We have observed the formation of stable structures ongoing after the removal of the perturbation. This suggests the formation of a stable far from- equilibrium state achieved trough the dissipation of energy subtracted to the environment. The supra molecular arrangement of liquid water depends on: Temperature Solutes Interfaces Electromagnetic signals Concentration (thank to Prof. Konovalov for discussion) [email protected] Glutamic acid • • • • pH<3.2 both carboxylic and amine groups are protonated and its ionic charge is -1 deprotonated species appear increasing pH isoelectric point = 3.2 pH, its ionic charge is 0 above pKa=9.7 the amino acid is fully deprotonated and its ionic charge is +2 [email protected] Glutamic acid speciation scheme pH When submitted to a weak ELF/static electromagnetic field the glutamic acid loses a proton The electric charge ranges from +1 in the fully protonated form to -2 according to the speciation scheme. pH=1.5 pH=11.8 [email protected] [email protected] When submitted to a magnetic field from 3 to 10 times higher than the geomagnetic field the kinetic of the reaction is increased up to 50% [email protected] Phenylalanine We have observed that the exposure to a weak magnetic field of an aqueous solution of L-Phe induces a measurable shift in the acid–base equilibrium. [email protected] Phenylalanine The exposure of L-Phe to the magnetic field has an effect similar to the exposure to NIR radiation, which is known to cause significant changes in the hydration properties of such molecules. Exposure to a static magnetic field 1 Gauss – 30 minutes [email protected] H 2O modifications aggregation pKa shift [email protected] We suggest that: the size and the hydrophobicity of the R group of the amino acids are responsible for the magnitude of the effect. the magnetic field acts as a “chaotrope” (disorder maker) agent, presumably acting upon the water supra-molecular structure. A major degree of aggregation between two amino acids is allowed whenever this layer is decreased by a magnetic field. [email protected] [email protected] Effect of the magnetic field on water 0 water . 3 Exposed water 0 Difference (X10) The magnetic field may induce a rearrangement in the structure of liquid water and modify the ratio between the two phases of water 0 A b s . 2 . 1 0 - 0 . 1 3 8 0 0 3 6 0 0 3 W a 4 v 0 e 0 n 3 u m b e 2 r 0 0 [ c m 3 - 1 0 0 0 2 8 0 0 ] Chaotrope* (disorder-maker) effect of the magnetic field (1888) Magnetic field Kosmotropes Chaotropes EMF cosmotrope effect effect -10 Effect on mM to M solutions It helps to form structures? Montagnier effect EMF emission MF EMF chaotrope ??? 0 logc [email protected] 1. Is the dynamical distribution of the two phases only function of the temperature? 2. Do the interaction with the environment affect the distributionkinds of the two Different of phases water ?are then possible according to the exchanged with the 3. Can one phase beinformation selectively stabilized? environment. The supra molecular liquid watersuitable is very sensitive Liquid waterstructure has aofstructure to to the environment including to the electromagnetic fields. transform those information in significance and The appearance of stable structures that survive even to the phase therefore in meaning. transition from liquid to solid state implies the existence of coherent spacetime dissipative structures, capable of exchange energy and matter with the environment and attaining a different level of organisation. Thank you for your attention