* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The history of the atomic model
Survey
Document related concepts
Transcript
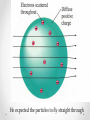
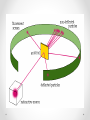
THE HISTORY OF THE ATOMIC MODEL DEMOCRITUS 4 6 0 B. C. T O 3 7 0 B. C. All matter consists of extremely small particles that cannot be divided. Called them “Atoms” from Greek atomos meaning indivisible ARISTOTLE 384 BC – 320 BC There is no limit to how many times you can divide matter. Aristotle: Four elements: Earth Water Air Fire For many centuries, people believed Aristotle’s theory of matter John Dalton 1766-1844 Dalton’s Theory: 1) All elements are composed of atoms 2) All atoms of the same element have the same mass, and atoms of different elements have different masses 3) Compounds contain atoms of more than one element 4) In a particular compound, atoms of different elements always combine in the same proportions Dalton made these wooden spheres to represent the atoms of different elements Dalton’s model of the atom is sometimes called the “solid sphere” or “Billiard ball” model Joseph John (J.J.) Thomson 1856-1940 Cathode Ray Tube • Thomson calculated the mass and charge of the particles in the beam • Negatively charged, 2000 times smaller than a hydrogen atom • Concluded that atoms must contain tiny, negatively-charge particles • Thomson had discovered the first subatomic particle, the electron Thomson’s “Plum Pudding” model Small electrons moving around in a sea of positive charge Ernest Rutherford 1871-1937 Designed an experiment in which small, positively charged particles were launched at a thin sheet of gold The experiment was performed by his students Geiger and Marsden He expected the particles to fly straight through Rutherford concluded that all of the positive charge and nearly all the mass of an atom was located in a tiny nucleus in the center. Electrons orbit around the nucleus like planets around the sun Rutherford’s model of the atom Neils Bohr 1885-1962 Proposed a theory that electrons can only be stable at certain distances from the nucleus The electrons in orbits farther from the nucleus have more energy Bohr Model of the atom Erwin Schrödinger Werner Heisenberg 1925 Schrodinger and Heisenberg: The Electron Cloud James Chadwick In 1932, discovered the neutron Neutrons are found in the nucleus They are about the same size as protons They have zero charge The History of the Atomic model Atoms are made up of protons, neutrons, and electrons. Protons have a positive charged, and neutrons have no charge. Protons and neutrons make up the nucleus in the center of the atom. Electrons have a negative charge and orbit the nucleus. The nucleus contains nearly all the atom’s mass. Protons and neutrons both have around 1800 times more mass than electrons. Electrons are located in the electron cloud, an area that surrounds and is much larger than the nucleus.