* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Infect Control Hosp Epidemiol
Transmission (medicine) wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Multiple sclerosis research wikipedia , lookup
Hepatitis C wikipedia , lookup
Urinary tract infection wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Sarcocystis wikipedia , lookup
Hepatitis B wikipedia , lookup
Multiple sclerosis signs and symptoms wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Neonatal infection wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Methicillin-resistant Staphylococcus aureus wikipedia , lookup
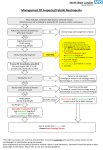
Surgical Site Infection: New Solutions to a Continuing Problem R. Lawrence Reed, II, MD, FACS, FCCM Professor of Surgery Loyola University Medical Center Director, SICU, Hines VA Medical Center Maywood, IL Surgical Site Infections (SSI) • Third most common nosocomial infection (14%–16%) • Most common nosocomial infection among surgical patients (38%) – 2/3 incisional – 1/3 organs or spaces accessed during surgery • 7.3 additional postoperative days at cost of $3,152 in extra charges Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Colonization vs Contamination – Definitions • Colonization – Bacteria present in a wound with no signs or symptoms of systemic inflammation – Usually less than 105 cfu/mL • Contamination – – – – Transient exposure of a wound to bacteria Varying concentrations of bacteria possible Time of exposure suggested to be < 6 hours SSI prophylaxis best strategy SSI – Definitions • Infection – – – – – Systemic and local signs of inflammation Bacterial counts ≥ 105 cfu/mL Purulent versus nonpurulent LOS effect Economic effect • Surgical wound infection is SSI LOS=length of stay. Superficial Incisional SSI Infection occurs within 30 days after the operation and involves only skin or subcutaneous tissue Skin of the incision Subcutaneous tissue Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Superficial incisional SSI Deep Incisional SSI Infection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and the infection involves the deep soft tissue (e.g., fascia and muscle layers) Deep soft tissue (fascia & muscle) Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Superficial incisional SSI Deep incisional SSI Organ/Space SSI Infection occurs within 30 days after the operation if no implant is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and the infection involves any part of the anatomy, other than the incision, which was opened or manipulated during the operation Organ/space Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Superficial incisional SSI Deep incisional SSI Organ/space SSI SSI – Risk Factors Operation Factors • • • • • • • • Duration of surgical scrub Maintain body temp Skin antisepsis Preoperative shaving Duration of operation Antimicrobial prophylaxis Operating room ventilation Inadequate sterilization of instruments • Foreign material at surgical site • Surgical drains • Surgical technique – Poor hemostasis – Failure to obliterate dead space – Tissue trauma Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. SSI – Risk Factors Patient Characteristics • Age • Diabetes – HbA1C and SSI – Glucose > 200 mg/dL postoperative period (<48 hours) • Nicotine use: delays primary wound healing • Steroid use: controversial • Malnutrition: no epidemiological association • Obesity: 20% over ideal body weight • Prolonged preoperative stay: surrogate of the severity of illness and comorbid conditions • Preoperative nares colonization with Staphylococcus aureus: significant association • Perioperative transfusion: controversial • Coexistent infections at a remote body site • Altered immune response Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Risk of Infection Bacterial dose Virulence Impaired host resistance Risk of Infection Bacterial dose Virulence Impaired host resistance RiskRisk of Surgical of Infection Infection Virulence Bacterial Bacterial dosedose Virulence Impaired Impaired host resistance host resistance SSI – Wound Classification • • • • Class 1 = Clean Class 2 = Clean contaminated Prophylactic antibiotics Class 3 = Contaminated indicated Class 4 = Dirty infected Therapeutic antibiotics Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. SSI – Risk Stratification NNIS Project 3 independent variables associated with SSI risk – Contaminated or dirty/infected wound classification – ASA > 2 – Length of operation > 75th percentile of the specific operation being performed NNIS=National Nosocomial Infections Surveillance. NNIS. CDC. Am J Infect Control. 2001;29:404-421. SSI – Wound Class vs NNIS Class Wound Class All NNIS 0 Clean Clean contaminated Contaminated Dirty infected 2.1% 3.3% 6.4% 7.1% 1.0% 2.1% N/A N/A 2.3% 4.0% 3.4% 3.1% 5.4% 9.5% 6.8% 8.1% N/A N/A 13.2% 12.8% All 2.8% 1.5% 2.9% 6.8% 13.0% NNIS. CDC. Am J Infect Control. 2001;29:404-421. NNIS 1 NNIS 2 NNIS 3 Campaign to Prevent Antimicrobial Resistance • Centers for Disease Control and Prevention • National Center for Infectious Diseases • Division of Healthcare Quality Promotion Clinicians hold the solution! Link to: Campaign to Prevent Antimicrobial Resistance Online Link to: Federal Action Plan to Combat Antimicrobial Resistance 12 Steps to Prevent Antimicrobial Resistance Among Surgical Patients • Step 1 – Prevent SSIs – Monitor and maintain normal glycemia – Maintain normothermia – Perform proper skin preparation using appropriate antiseptic agent and, when necessary, hair removal techniques – Think outside the wound to stop surgical site infections CDC. Available at http://www.cdc.gov/drugresistance/healthcare/surgery/12steps_surgery.htm. Accessed July 16, 2004. Opportunity to Prevent SSI • An estimated 40%–60% of SSIs are preventable • Overuse, underuse, improper timing, and misuse of antibiotics occurs in 25%–50% of operations Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Principles of Antibiotic Prophylaxis Preop administration, serum levels adequate throughout procedure with a drug active against expected microorganisms. High Serum Levels 1. Preop timing 2. IV route 3. Highest dose of drug During Procedure 1. Long half-life 2. Long procedure– redose 3. Large blood loss– redose Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Duration 1. None after wound closed 2. 24 hours maximum Surgical Site Infection (SSI) Mangram AJ et al. Infect Control Hosp Epidemiol. 1999;20:250-278. Surgical Infection Prevention Project Medicare Quality Improvement Community Clinical Infectious Diseases 2004 June; 38:1706–15 National Data Collection • State-level baseline description from random sample of 788 cases per state • Data collected from records by two professional clinical data abstraction centers • Abstraction tool for hospitals is available….Is JCAHO compatible Surgical Infection Prevention Preliminary Results N (%) Number of cases reviewed 39,086 (100) General Exclusions Surgery of interest not performed Infection present pre-operatively Missing antibiotic dates and times Patient on antibiotics prior to admission Patient on antibiotics for more than 24 hours pre-op Other 205 (0.52) 1,817 (4.7) 2 (0.01) 1,461 (3.74) 1,432 (3.66) 36 (0.09) Cases eligible for analysis 34,133 (87.3) Discontinuation of Antibiotics 100 88 85.8 100 90.7 79.5 60 60 50.7 40.7 40 40 26.2 22.6 14.5 20 20 10 9.3 6.3 6.2 2.7 2.2 96 > -9 6 >8 4 -8 4 >7 2 -7 2 >6 0 -6 0 >4 8 -4 8 >3 6 -3 6 >2 4 >1 2 le or -2 4 0 ss 0 Hours After Surgery End Time Patients were excluded from the denominator of this performance measure if there was any documentation of an infection during surgery or in the first 48 hours after surgery. Cumulative Percent 80 73.3 12 Percent 80 Most Common Pathogens Associated With Nosocomial Infections (NNIS 1989–1998) Medical & Surgical Combined Relative Percentage by Site of Infection Pathogen All Sites n=235,758 BSI n=50,091 Pneumonia n=64,056 SSI n=22,043 Coag-neg Staph S aureus 14.3 11.4 39.3 10.7 2.5 16.8 13.5 12.6 Enterococci spp. P aeruginosa Enterobacter spp. E coli C albicans K pneumoniae Others 8.1 9.9 7.3 7.0 6.6 4.7 30.7 10.3 3.0 4.2 2.9 4.9 2.9 21.8 1.9 16.1 10.7 4.4 4.0 6.5 37.1 14.5 9.2 8.8 7.1 4.8 3.5 26.0 BSI=bloodstream infection; SSI=surgical site infection. Fridkin SK et al. Clin Chest Med. 1999;20:303-316. Predominance of S aureus in Skin and Skin Structure Infections (SSSIs) SENTRY – US and Canada 2000 Other 17.3% MSSA 30.9% Klebsiella 5.1% Enterobacter 5.8% E coli 7.0% Enterococci 8.2% 10.8% P aeruginosa N=1,404 isolates Rennie RP et al. Diagn Microbiol Infect Dis. 2003;45:287-293. MRSA ~15% Progression of Methicillin Resistant S aureus – United States Resistant isolates (%) 60 13% 57.1% 55.3% 50 40 30 20 10 20 00 20 02 99 98 97 96 95 94 93 92 91 90 89 88 87 19 75 0 CDC. MMWR. 1997;46:624-628, 635. (1975 data); Lowy FD. N Engl J Med. 1998;339:520-532. (1987-1997 data); CDC. NNIS System Report, January–November 1998. (1998 data); CDC. NNIS System Report, January 1990–May 1999, issued June 1999. Am J Infect Control. 1999;27:520-532. (1999 data); CDC. NNIS System Report, January 1992–June 2001. Am J Infect Control. 2001;29:404-421. (2000 data); NNIS. CDC. Am J Infect Control. 2003;31:481-498. Impact of MRSA on SSI Median Hospital Charges 100,000 90,000 80,000 70,000 60,000 50,000 40,000 30,000 20,000 10,000 0 92,363 52,971 29,455 • N=479 patients • MRSA greater 90-d mortality vs MSSA (adjusted odds ratio, 3.4; 95% CI: 1.5–7.2) • MRSA longer LOS after infection (median additional days=5; P<0.001) • MRSA associated with greater hospital charges (1.19-fold increase in hospital charges, P=0.03) Engemann JJ et al. Clin Infect Dis. 2003;36:592-598. Vascular SSI • Retrospective review (1993–2000) – Leicester Royal Infirmary, United Kingdom – 172 patients MRSA-positive (4.4% of total) – 75 infected, 97 colonized • Proportion of wound/graft infections caused by MRSA has increased – 4% in 1994, increased to 63% in 2000 • All patients with aortic graft infection died • All patients with infected prosthetic infrainguinal bypass required amputations Nasim A et al. Eur J Vasc Endovasc Surg. 2001;22:211-214. MRSA in Orthopedic SSI • • • • • Prospective study, London, United Kingdom 12-month study, January through December 2000 Total of 1,879 patients admitted, 121 screened 1.6% of total with MRSA infection/colonization Higher risk for MRSA infection – Hip surgery – Emergency surgery for femoral neck fracture – Presence of wound • MRSA infection – increased hospital LOS (88 vs 11 days) • 41% of positive patients still carried MRSA on discharge Tai CC et al. Int Orthop. 2004;28:32-35. MRSA in Cardiac Surgery • 3,443 CABG patients, all received antimicrobial prophylaxis • June 1997 through December 2000 • Sternal SSI developed in 122 (3.5%) – 71 (58.2%) were superficial SSI – 51 (41.8%) were deep SSI • Gram-positive cocci were most frequently recovered (81%) • S aureus was the most frequently isolated pathogen (49%) • S aureus bacteremia occurred in 18% and was significantly associated with deep SSI (P=0.002) CABG=coronary artery bypass grafting. Sharma M et al. Infect Control Hosp Epidemiol. 2004;25:468-471. Impact of MRSA in Cardiac Surgery • Retrospective review (41 patients) – Poststernotomy S aureus mediastinitis – MRSA: 15 patients – MSSA: 26 patients • Logistic regression analysis: MRSA was the only independent risk factor for increased mortality, P=0.04 Survival Rates 90 80 70 60 50 1 month 1 year 3 years 40 30 20 10 0 Mekontso-Dessap A et al. Clin Infect Dis. 2001;32:877-883. MRSA MSSA • 4,030 patients enrolled, 3,864 ITT patients • PRDBPCT, intranasal mupirocin • 891 patients (23.1%) had S aureus in anterior nares – 444 mupirocin, – 447 placebo • S aureus SSI: – 2.3% mupirocin – 2.4% placebo Percent of patients with S aureus (%) Nasal Mupirocin and SSI All postoperative S aureus Infections 7.7 P=0.02 4 Mupirocin Placebo ITT=intent-to-treat; PRDBPCT=prospective, randomized, double-blind placebo-controlled trial. Perl TM et al. N Engl J Med. 2002;346:1871-1877. Surgical Wound Management SSI Prophylaxis in MRSA-Colonized Patient • Must use same principles • Drug choice difference • MRSA drugs – – – – Vancomycin Quinupristin/dalfopristin* Linezolid Daptomycin *Not FDA approved for MRSA. Vancomycin • Bactericidal glycopeptide – Discovered in 1956 – Produced by Streptococcus orientalis, an actinomycete isolated from soil samples from Indonesia & India • Introduced clinically in 1958 • Quickly overshadowed by less toxic antistaphylococcal penicillins and cephalosporins • Re-emergence as an important antibiotic in 1980s & 1990s Historical Yearly Usage of Vancomycin 2001: 1.8 million courses of vancomycin annually in U.S. 30 million doses of vancomycin estimated Kirst HA et al. Antimicrob Agents Chemother. 1998;42:1303-1304; NNIS. Am J Infect Control. 2001;29: 404-421. 12 Steps to Prevent Antimicrobial Resistance Among Surgical Patients • Step 9. Know when to say “no” to vanco – Vanco should be used to treat known infections, not for routine prophylaxis – Treat staphylococcal infection, not contaminants or colonization – Consider other antimicrobials in treating MRSA CDC. Available at http://www.cdc.gov/drugresistance/healthcare/surgery/12steps_surgery.htm. Accessed July 16, 2004. Vancomycin Tissue Penetration – Below the mean MICs for many strains of staphylococci 4.5 4 Level (mg/L) • 33 open-heart surgery patients, mean vancomycin concentrations after 15 mg/kg IV dose 3.5 Tissue Level (mg/L) 3 MIC (mg/L) 2.5 2 1.5 1 0.5 0 Cardiac Valve Myocardium Tissue MIC=minimum inhibitory concentration. Daschner FD et al. J Antimicrob Chemother. 1987;19:359-362. Fat CNS: <10% Epithelial lining fluid3: 18% Sternal Bone1: 57% Heart Valve4: 12% Lung tissue2: 17%–24% Vancomycin Penetration Bone5: 7%–13% Fat4: 14% Muscle4: 9% 1. Massias L et al. Antimicrob Agents Chemother. 1992;36:2539-2541; 2. Cruciani M et al. J Antimicrob Chemother. 1996;38:865-869. 3. Lamer C et al. Antimicrob Agents Chemother. 1993;37:281-286; 4. Daschner FD et al. J Antimicrob Chemother. 1987;19:359-362; 5. Graziani AL et al. Antimicrob Agents Chemother. 1988;32:1320-1322. Quinupristin/Dalfopristin (Synercid®) • Streptogramin class related to macrolide-lincosamides – Quinupristin is a Group B streptogramin – Dalfopristin is a Group A streptogramin • Activity against: – MSSA – potently bactericidal – Streptococcus pneumoniae (including PRSP) – potently bactericidal – MRSA – moderately active – E faecium – moderately active against most E faecium strains – NO activity against E faecalis PRSP=penicillin-resistant Streptococcus pneumoniae. Synercid® IV (quinupristin/dalfopristin for injection) [package insert]. Bristol, Tenn: Monarch Pharmaceuticals, Inc; 2002. Quinupristin/Dalfopristin (Synercid®) • Central line access used to decrease incidence of infusion-related venous irritation • 3%–30% incidence of severe myalgias and arthralgias • Resistance has already been reported • Bacteriostatic • Does not have indication for pneumonia – Did not perform as well as vancomycin Synercid® IV (quinupristin/dalfopristin for injection) [package insert]. Bristol, Tenn: Monarch Pharmaceuticals, Inc; 2002. Daptomycin (Cubicin™) • • • • • • • • Lipopeptide natural product Activity in Gram-positive organisms Distinct mechanism of action Rapidly bactericidal in vitro and in vivo No mechanisms of resistance identified No cross-resistance with other antibiotics Safety profile similar to comparators Once-daily IV dosing Cubicin™ (daptomycin for injection) [prescribing information]. Lexington, MA: Cubist Pharmaceuticals; September 2003. Linezolid (ZYVOX®) • • • • • • • • An oxazolidinone: a novel antimicrobial class 100% oral bioavailability Equivalent dosing oral/IV No dose adjustment in renal failure Bacteriostatic No cross-resistance with other antibiotics Reversible thrombocytopenia with prolonged use Binds selectively to the 50S ribosomal subunit – Inhibits the formation of a functional initiation complex ZYVOX® (linezolid injection, tablets, and oral suspension) [package insert]. Kalamazoo, Mich: Pharmacia & Upjohn, a Pfizer Company; revised June 2004. Average Steady-State Plasma Linezolid Concentrations After Oral Administration of 400 or 600 mg Twice Daily Linezolid concentration (μg/mL) 20 18 600 mg BID 16 400 mg BID 14 MIC-90 Staph 12 MIC-90 Entero 10 MIC-90 Strep 8 6 4 2 0 0 5 10 15 20 Time After Last Dose (hours) Linezolid Research Update. Denver, Colo: Infectious Diseases Society of America; November 13, 1998. CNS1: 70%* Epithelial lining fluid4: 450% Alveolar cells4: 15% Saliva2: 120% Linezolid Penetration Bone3: 40%–60% Sweat2: 55% Skin Blister Fluid5: 100% 1. Cottagnound et al. J Antimicrob Chemother. 2000;46:981-985; 2. ZYVOX® (linezolid injection, tablets, and oral suspension) [package insert]. Kalamazoo, Mich: Pharmacia & Upjohn, a Pfizer Company; revised 2003; 3. Lovering AM et al. J Antimicrob Chemother. 2002, 50:73-77; 4. Conte JE et al. Antimicrob Agents Chemother. 2002;46:1475-1480; 5. Gee T. Antimicrob Agents Chemother. 2001;45:1843-1846. Comparison of Tissue Concentrations (% Tissue/Serum) Tissue Vancomycin Linezolid Bone 7%–13%1 60%8 Cerebral Spinal Fluid 0%–18%2,3 70%9 Epithelial Lining Fluid (Lung) 11%–17%4,5 450%9 ---- 104%10 Muscle ~30%6 94%8 Peritoneal dialysis fluid ~20%7 61%11 Inflammatory Blister Fluid 1. Graziani AL et al. Antimicrob Agents Chemother. 1988;32:1320-1322; 2. Matzke et al. Clin Pharmacokinet. 1986;11:257-282; 3. Albanese J et al. Antimicrob Agents Chemother. 2000;44:1356-1358; 4. Georges H et al. Eur J Clin Microbiol Infect Dis. 1997;16:385-388; 5. Lamer C et al. Antimicrob Agents Chemother. 1993;37:281-286; 6. Daschner FD et al. J Antimicrob Chemother. 1987;20:776-782; 7. Blevins RD et al. Antimicrob Agents Chemother. 1984;25:603-606; 8. Lovering AM et al. J Antimicrob Chemother. 2002;50:73-77; 9. Conte JE et al. Antimicrob Agents Chemother. 2002;46:1475-1480; 10. Gee T et al. Antimicrob Agents Chemother. 2001;45:1843-1846; 11. Gendjar SR et al. 2001 ASN/ISN World Congress of Nephrology; 2001; San Francisco, Calif. Abstract 550865. Complicated Skin and Soft Tissue Infection (cSSTI) Treatment • Staph most common cause • Staph resistance continues to increase – 57.1% in 2002 • Treatment for MRSA cSSTI prior to 2000 – Vancomycin – Quinupristin/dalfopristin* • New alternatives for treatment of MRSA cSSTI – Linezolid – Daptomycin *Not FDA approved for MRSA. NNIS. CDC. Am J Infect Control. 2003;31:481-498. Quinupristin/Dalfopristin† (Q/D) Efficacy • Design: 2 randomized, open-label, controlled clinical trials in cSSSI – Study 1: Q/D (7.5 mg/kg q12h IV) vs oxacillin (2 g q6h IV)* – Study 2: Q/D (7.5 mg/kg q12h IV) vs cefazolin (1 g q8h IV)* Efficacy in the Clinically Evaluable Population† Q/D (n=450) Comparator (n=443) Study 1 (US) 49.5% 51.9% Study 2 (International) 66.4% 64.2% Postoperative infections‡ 14/38 (36.8%) 24/42 (57.1%) Traumatic wound infections‡ 33/55 (60.0%) 33/55 (60.0%) *Vancomycin 1 g q12h IV could be substituted if the pathogen was suspected or confirmed methicillin-resistant Staphylococcus or the patient was allergic to penicillin, cephalosporins, or carbapenems. †Patients cured or improved. ‡Results are combined from the 2 clinical trials. Statistical conclusions could not be reached due to the small number of patients in the subsets. †Not FDA approved for MRSA. Synercid® IV (quinupristin/dalfopristin for injection) [package insert]. Bristol, Tenn: Monarch Pharmaceuticals, Inc; 2002. Quinupristin/Dalfopristin† (Q/D) Efficacy • Design: 2 randomized, open-label, controlled clinical trials in cSSSI Summary of Clinical and Microbiologic Results* Q/D (n=450) Comparator (n=443) Clinical efficacy† 68.2% 70.7% Microbiologic eradication‡ 66.6% 77.7% MSSA 64.3% 76.6% MRSA 77.8% 50.0% Gram-positive cocci only 56.3% 69.7% *Results are combined from the 2 clinical trials. †Patients cured or improved in the clinically evaluable population. ‡Overall and by-pathogen bacteriologic eradication rates in the microbiologically evaluable population. cSSSIs=complicated skin and skin structure infections. †Not FDA approved for MRSA. Nichols RL et al. J Antimicrob Chemother. 1999;44:263-273. Linezolid vs Vancomycin for cSSTI Presumed or Known to Be Caused by MRSA • Study design: Open-label, randomized, comparatorcontrolled, multicenter, multinational clinical study • Population: 1,200 hospitalized adult patients with cSSTI • Treatment arms: Linezolid (oral or IV) 600 mg every 12 hours OR Vancomycin (IV only) 1 g every 12 hours If MSSA, vancomycin could be switched to oxacillin/nafcillin/flucloxacillin (IV only) 1–2 g q6h or dicloxacillin (oral) 500 mg q6h 4- to 14-day treatment duration Weigelt JA et al. Infectious Diseases Society of America, 2003, poster 314. San Diego, CA. Linezolid vs Vancomycin for cSSTI Clinical Cure Rates in Clinically Evaluable Subset Linezolid 600 mg q12h IV/PO 100 94 Vancomycin 1g q12h IV* 90 Clinical cure rate (%) 80 P=0.023 60 40 20 0 436/462 394/436 Weigelt JA et al. Infectious Diseases Society of America, 2003, poster 314. San Diego, CA. Linezolid vs Vancomycin for cSSTI Clinical Cure Rates in MRSA Subgroup Linezolid 600 mg q12h IV/PO Clinical cure rate (%) 100 Vancomycin 1g q12h IV* 94 84 80 *P=0.011 60 40 20 0 126/134 112/134 Weigelt JA et al. Infectious Diseases Society of America, 2003, poster 314. San Diego, CA. Linezolid Reduces LOS vs Vancomycin in cSSTI due to MRSA Mean LOS (days) Study Sample (linezolid/vancomycin) Linezolid Vancomycin P Value ITT (592/588) 7.4 9.8 <0.0001 CE (491/472) 7.4 9.9 <0.0001 ME (349/334) 7.6 9.8 <0.0001 MRSA (143/146) 8.1 10.7 0.0026 CE=clinically evaluable; ITT=intent-to-treat; LOS=length of stay; ME=microbiologically evaluable. Weigelt JA et al. Infectious Diseases Society of America; 2003, poster 315. San Diego, CA. Linezolid vs Vancomycin for cSSTI IV Antibiotic Treatment Days Duration of IV treatment (days) Linezolid Vancomycin 14 12.6 12 10 9.9 9.1 9 9.9 8 6 4 1.9 2 1.9 2 1.8 2 0 ITT MITT CE ME MRSA Sample population CE=clinically evaluable; ITT=intent-to-treat; ME=microbiologically evaluable; MITT=modified intent-to-treat. Weigelt JA et al. Infectious Diseases Society of America, 2003, poster 315. San Diego, CA. Cost Effectiveness of Linezolid vs Vancomycin in cSSTI $5,187 6000 5000 C O S T ($) $4,143 CI (4,691–5,714) CI (3,750–4,576) 4000 3000 2000 1000 0 Linezolid Vancomycin 2003 Per diem hospital cost, administration of IV therapy, wholesale acquisition cost Fleming T, ed. Red Book. 2004 edition. Montvale NJ: Thompson PDR;2004. Linezolid vs Vancomycin for Surgical Site Infection (SSI ) Total Patients With cSSTI 1,200 Total Patients With SSI 135 Linezolid 66 Vancomycin 69 -Weigelt J et al.: Am J Surg 2004;188:760-766. Linezolid vs Vancomycin in SSI Study Population Study Population Linezolid (n, %) Vancomycin (n, %) All patients 66 (100) 69 (100) MRSA 34 (52) 31 (45) Baseline demographics: No significant difference Baseline comorbidities/MRSA risk factors: No significant difference -Weigelt J et al.: Am J Surg 2004;188:760-766. Linezolid vs Vancomycin in SSI: Clinical Cure Rates at TOC Linezolid 600 mg q12h IV/PO Clinical cure rate (%) 100 Vancomycin 1g q12h IV 98 87 80 P=0.06 60 40 20 0 52/53 47/54 -Weigelt J et al.: Am J Surg 2004;188:760-766. Linezolid vs Vancomycin in SSI: Microbiological Cure Rates at TOC Linezolid 600 mg q12h IV/PO Clinical cure rate (%) 100 Vancomycin 1g q12h IV 84 80 P=0.007 60 58 40 20 0 41/49 28/49 -Weigelt J et al.: Am J Surg 2004;188:760-766. Linezolid vs Vancomycin in SSI: Microbiological Cure Rates at TOC in MRSA Pts. Linezolid 600 mg q12h IV/PO Clinical cure rate (%) 100 Vancomycin 1g q12h IV 87 80 60 P=0.002 48 40 20 0 26/30 14/29 -Weigelt J et al.: Am J Surg 2004;188:760-766. Daptomycin for cSSSIs • Phase III: 2 international, multicenter, randomized, double-blind (evaluator blinded) studies (Studies 9801 & 9901): – Daptomycin (4 mg/kg IV qd) vs 1 of 2 comparators: • Vancomycin (1 g q12h) • Synthetic penicillin (4–12 g/d in 4 daily doses) • Primary endpoint was safety and efficacy • Both studies demonstrated equivalence of daptomycin to the comparator Arbeit RD et al. Clin Infect Dis. 2004;38:1673-1681. Daptomycin Efficacy • • Design: 2 randomized, multinational, multicenter investigator-blinded studies Daptomycin 4 mg/kg IV q24h or vancomycin 1 g IV q12h or a semisynthetic penicillin (nafcillin, oxacillin, cloxacillin, flucloxacillin) Clinical Success Rate: CE Population Type of Infection Daptomycin Comparator* No. of Pts (Success rate %) No. of Pts (Success rate %) Wound infection 169 (84%) 180 (87%) Major abscess 102 (92%) 92 (88%) Ulcer infection 47 (66%) 56 (70%) Other infection† 47 (79%) 58 (83%) *Comparator was vancomycin or a semisynthetic penicillin. †Other infections included complicated cellulitis, major abscess, or traumatic wound infection. Arbeit RD et al. Clin Infect Dis. 2004;38:1673-1681. Comparison of MRSA Antimicrobials Vancomycin Quinupristin/ Dalfopristin Daptomycin Linezolid FDA approved for MRSA Multiple cSSSI (not MRSA) cSSSI cSSSI and pneumonia Route(s) Parenteral Parenteral (central?) Parenteral Parenteral, oral Dosing Variable depending upon renal function q8-12h QD BID Sales pitch “Cheap” “Works when vancomycin won’t” “Faster cure” (rapidly cidal) “Gets patients home” Disadvantages Toxicity, resistance (VRE, VISA, VRSA) Infusion site inflammation, myalgias, arthralgias, and resistance Limited indications, acquisition cost, myalgia, not effective for pneumonia Unfamiliarity and cost, reversible hematologic abnormalities, resistance (ie, VRE with prolonged use) Advantages Familiarity Alternative to vancomycin Potential for less resistance Oral dosingremoval of catheters, early discharge, evidence of superiority to vancomycin in cSSTI Summary • SSI is a preventable morbidity • Gram-positive organisms are the primary pathogens – MRSA increasing • Treatment alternatives in MRSA SSIs and cSSTIs – – – – Vancomycin Linezolid Daptomycin Quinupristin/dalfopristin* *Not FDA approved for MRSA.