* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electric Current
Survey
Document related concepts
Transcript
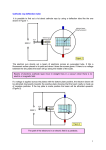
G482 Module 1 – Electric Current Electric Current Electric current is a net flow of charged particles. These charged particles can be electrons (as in a wire), or positive or negative ions (such as in an ionic solution). There are a few ways to get charged particles to flow: • We can push free electrons around a conducting wire using a potential difference. • Or we can push ions in an ionic solution between two electrodes by applying a potential difference. • Or we can fire a beam of charged particles (usually electrons) through a vacuum. [demo – electron beam causing electric current] [demo – Van Der Graaf generator & fluorescent tube] Whichever method we use, we are moving charged particles from one place to another. Electric Current in Metals Metals contain free electrons; these are electrons that are not specifically bound to one atom and are free to move around inside the metal. Application of a potential difference will cause a net flow of these free electrons in one direction, this is an electric current. Electric Current in Electrolytes Electrolyte – a substance containing free ions Electrolytes contain free ions, both positive and negative, which are attracted or repelled by a voltage (depending on their charge). The movement of these free ions is what causes an electric current to flow in an electrolyte such as salt water. [demo – conduction of copper sulphate solution in water] [demo – conduction of salt water] When salt (NaCl) is put into solution the sodium ions disassociate from the chlorine ions and you end up with Na+ ions and Cl- ions floating around. The Na+ ions are attracted to the negative electrode and the Cl- ions are attracted to the positive electrode, this movement of charged particles is an electric current. Conventional Current vs Electron Flow As you know, electrons are negatively charged particles; this means they are repelled by a negative charge and attracted to a positive charge. You also know that conventional electric current flows from positive to negative. Actually, convention is wrong. It came about before people knew what electrons were and became so widespread that it was impossible to change, therefore our convention is opposite to reality but it doesn’t really matter. Electron flow direction Traditional current direction + to - - to + QUESTION: 2.5 Which one of the following situations results in a conventional electric current that flows westward? a beam of protons moves eastward a electric dipole moves westward a beam of electrons moves westward a beam of electrons moves eastward a beam of neutral atoms moves westward Conventional Electron flow current flow Answer As I am sure you know the conventional direction of electric current is the movement of charges from positive to negative – in other words a flow of positive charges. We need to look at the options and see if we can find positive charges moving to the west – or of course negative charges moving to the east. In your options the only possibility is: a beam of electrons moves eastward The others are no good because: a beam of protons moves eastward - positive charges moving east a electric dipole moves westward - a dipole has no net charge a beam of electrons moves westward - negative charges moving west a beam of neutral atoms moves westward - neutral atoms have no net charge Charge, Current and Time As you should remember from GCSE: Charge = current x time. We’re going to make a very minor addition at A-Level and write: ΔQ = I Δt Where: ΔQ = amount of charge that has flowed (Coulombs / C) I = current (Amps / A) Δt = time taken for charge to flow (seconds / s) Note that the symbol Δ (a Greek capital delta) means “change in” and should always be kept together with its partner letter. Don’t start trying to separate the deltas from the letters! Worked Example Calculate the current flowing when 12 C moves past a point in 2.0 s. ΔQ = I Δt 12 = I x 2 I=6A Easy. 1 Coulomb = the amount of electric charge transported by a steady current of 1 amp in 1 second. The charge on one electron = -1.6 x 10-19 C Use of an Ammeter An ammeter can be used to measure the current in an electric circuit. You simply connect it in series with the circuit you want to measure. A . Kirchoff’s First Law The algebraic sum of the currents flowing through a junction is zero. Currents approaching the junction are + while currents leaving the junction are -. This sounds a lot more complicated than it really is. I I1 I2 It just means that in this case I = I1 + I2. The total current flowing into a junction has to leave it. This means that no charge can be built up in the junction and that charge is conserved. You may have encountered this in a different form: current splits in a series circuit. All we’re doing here is assigning a name and numbers to that rule. http://regentsprep.org/Regents/physics/phys03/bkirchof1/default.htm Drift Velocity Electrons in a wire don’t all move in the same direction, they tend to bezz about all over the place, even in the presence of a potential difference. However, on average they will move towards the positive terminal of the battery. This average movement is known as drift velocity and we can calculate it like this: Consider what happens to a single electron, e, starting at P and moving at v m/s. In 1 second, the electron will move v metres, i.e. from P to Q. volume of wire in section PQ = Av If there are n free electrons per m3 in the wire: Total number of electrons in PQ = Anv Each electron has a charge of e, so: Total free charge in PQ = Anev Since in the second we are considering the electron shown moves from P to Q, all of the electrons that were in PQ to start with will move past Q in that second: Charge passing Q in 1 s = I = Anev In some situations the charge carriers may not be electrons. A more general form of the equation, for particles of charge q, is: I = Anvq Conductors, Semiconductors and Insulators Conductors, such as all metals, contain free electrons. These free electrons can carry electrical charge wherever they go and are free to do so. Charge can flow easily through conductors and always becomes evenly distributed across the conductor’s surface when any external electric field is removed. Conductors have a high number of electrons per m3 (high n from the I = Anev formula) Insulators do not contain free electrons, so charge cannot move through them. When a static charge is introduced to an insulator it stays in one place. This is why balloons stick to walls when charged and tinfoil balls do not. Insulators have a low number of electrons per m3 (low n) Semiconductors, such as silicon, will only conduct if they are given sufficient energy. Electrons are usually tightly bound to the nucleus as in an insulator; however, the electrons can be freed if sufficient energy is provided. This allows the material to conduct under certain conditions. Semiconductors are usually ‘switched on’ by applying a voltage to them. Though heating is also effective. Semiconductors have a value of n which is between that of a conductor and an insulator.