* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Management of Atrial Fibrillation in the ICU
Survey
Document related concepts
Transcript
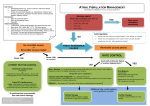
ANALYTIC REVIEWS The Management of Atrial Fibrillation in the ICU Paul E. Marik, MD, FCCM, and Gary P. Zaloga, MD, FCCM Marik PE, Zaloga GP. The management of atrial fibrillation in the ICU. J Intensive Care Med 2000;15:181–190. Atrial tachyarrhythmias are the most frequent arrhythmias occurring in ICU patients, being particularly common in patients with cardiovascular and respiratory failure. Unlike ambulatory patients in whom atrial fibrillation/flutter (AF) is likely to be short lived, in the critically ill these arrhythmias are unlikely to resolve until the underlying disease process has improved. Urgent cardioversion is indicated for hemodynamic instability. Treatment in hemodynamically stable patients includes correction of treatable precipitating factors, control of the ventricular response rate, conversion to sinus rhythm, and prophylaxis against thromboembolic events in those patients who remain in AF. Diltiazem is the preferred agent for rate control, while procainamide and amiodarone are generally considered to be the antiarrhythmic agents of choice. From the Department of Internal Medicine, Washington Hospital Center, Washington, DC. Received Nov 29, 1999, and in revised form Jan 31, 2000. Accepted for publication Feb 4, 2000. Address correspondence to Dr Marik, Department of Internal Medicine, RM-2A-68, Washington Hospital Center, 110 Irving St. NW, Washington, DC 20010–2975, or e-mail: [email protected]. Atrial tachyarrhythmias are the most common arrhythmias occurring in ICU patients, with a reported prevalence of 28% [1]. Atrial fibrillation is the most common atrial arrhythmia, followed by atrial flutter and multifocal atrial tachycardia (MAT). Since the etiology and management strategies of atrial fibrillation and atrial flutter overlap, for the purposes of this discussion they will be considered as one entity (AF). Patients who develop AF in the ICU have a worse prognosis than those who remain in sinus rhythm (SR); however, the attributable mortality of AF is unclear [1]. Pathophysiology Atrial fibrillation is a rhythm in which atrial activation is disorganized and the atria do not contract effectively. It is probably caused by a variant of reentry in which one or more activation wavefronts travel in endlessly varying paths, giving rise to subsidiary wavelets [2–4]. Atrial enlargement helps sustain AF by accommodating more wavelets [5]. AF is particularly common in ICU patients with cardiovascular disorders, respiratory failure, and sepsis [1,6]. The etiology is largely multifactorial, with hypoxia, electrolyte disturbances (hypokalemia, hypomagnesemia), myocardial ischemia, anemia, increased sympathetic tone, ‘‘cardiotonic drugs,’’ and atrial distention being implicated [1]. Pulmonary hypertension with right atrial distention may be an important precipitant in patients with respiratory failure and sepsis [6]. Underlying cardiovascular disease, diabetes, and advanced age increase the risk of developing atrial arrhythmias [1]. Drugs such as catecholamines and theophylline increase the risk of developing atrial tachyarrhythmias and increase the ventricular response, making rate control more difficult [7–10]. Patients with sudden onset of AF and no obvious underlying cause should be investigated for the possibility of pulmonary embolism. The natural history of untreated AF in critically ill patients has not been studied. However, it is unlikely that spontaneous conversion will occur until the underlying disease process has improved or resolved [6]. This contrasts with noncritically ill patients admitted with recent onset AF, Copyright q 2000 Blackwell Science, Inc. 181 182 Journal of Intensive Care Medicine Vol 15 No 4 July/August 2000 in whom spontaneous conversion will occur in up to 67% of patients [11]. The development of AF in critically ill patients is frequently associated with increased pulmonary capillary wedge pressure, decreased cardiac output, pulmonary hypertension, and worsening respiratory failure. In addition, AF increases morbidity and mortality in critically ill patients as a result of systemic emboli. In critically ill patients, the development of AF is often associated with significant hemodynamic compromise [6]. The decrease in cardiac output that accompanies AF may cause or exacerbate tissue hypoxia. AF results in a decrease in cardiac output as a consequence of reduced diastolic filling time and loss of atrioventricular synchrony [12]. Loss of atrioventricular synchrony compromises stroke volume and cardiac output to a variable degree depending upon ventricular compliance, venous filling pressure, ventricular rate, and other hemodynamic factors. The importance of atrioventricular synchrony is illustrated in the study of Donovan et al. [13], who demonstrated an increase in the mean cardiac output from 4.5 to 5.3 L/min when critically ill patients were switched from ventricular demand pacing to atrioventricular pacing. studies of the treatment of AF in ICU patients. The treatment algorithm outlined in this article is based on the synthesis of data from the above cited studies [12,14–16], as well as those randomized controlled studies that have enrolled patients presenting to the hospital with new onset AF [17–27]. AF is a common complication in postoperative coronary artery bypass patients; this is a distinct clinical problem which will not be reviewed in this article. The components of the acute management of AF include assessment of the need for urgent cardioversion, correction of treatable precipitating factors, control of the ventricular response rate, conversion to sinus rhythm, and prophylaxis against thromboembolic events in those patients who remain in AF. Rate control and correction of treatable precipitating factors is essential in all patients. Conversion to sinus rhythm may be important in patients who remain in AF, as AF is usually associated with a significant decrease in cardiac output which may be poorly tolerated in critically ill patients. Furthermore, prompt termination of AF may obviate the need for anticoagulation. In addition, prolonged AF causes electrophysiologic alterations in cardiac tissue that can perpetuate the arrhythmia, a process known as electrophysiologic remodeling [28]. Management of AF Urgent Cardioversion. Electrical cardioversion is a time-honored, highly effective method for converting AF to sinus rhythm. It is probably underutilized in ICU patients. Urgent cardioversion is indicated when the ventricular response is greater than 130 beats/min in association with angina, acute cardiac failure, hypotension [mean atrial pressure (MAP) < 70 mmHg or decrease in MAP > 15 mmHg], or indices of inadequate tissue perfusion [28–30]. Patients should be well sedated and given an analgesic prior to cardioversion. The combination of midazolam (2–10 mg) and fentanyl (50–150 mg) may be used in nonintubated patients. Ketamine (a neuroleptic anesthetic agent with potent analgesic properties) is an alternative in patients in whom respiratory depression [e.g., chronic obstructive pulmonary disease (COPD)] needs to be avoided. It should be emphasized that in ICU patients AF is a multiple disease process whose management may differ depending on the underlying etiology (i.e., sepsis, pericarditis, acute myocardial infarction, acute respiratory failure, pulmonary embolism, etc.). However, on the basis that most episodes of AF in critically ill patients are associated with some degree of hemodynamic compromise, we believe that all patients with this arrhythmia should be treated. Despite the high prevalence of AF in ICU patients, only two prospective studies [14,15], and two retrospective studies [12,16] (a total of 91 patients) have reported on the management of AF in critically ill ICU patients (Table 1). Of note, there have been no placebo-controlled, prospective randomized Table 1. Trials of Acute AF in ICU Patients Reference Year Number of Patients P/R* 14 15 16 12 1993 1995 1998 1996 24 21 38 8 P P R R *P 4 prospective; R 4 retrospective. Drugs Studied Procainamide versus amiodarone Magnesium versus amiodarone Amiodarone Amiodarone Conversion Success Rate (%) 71 versus 70 78 versus 50 48 87 Marik and Zaloga: Atrial Fibrillation in the ICU The combination of propofol and fentanyl is useful for short-duration hypnosis and analgesia for cardioversion in intubated patients. The aim of electrical therapy is to provide a current through the myocardium to change enough myocardial cells to the same electrical state for a sufficient time to break the reentrant circuits or produce electrical homogeneity. This allows a stable rhythm to be reestablished from the sinus node. Animal experiments have shown that the period of homogeneity needs to be 4–12 msec. More organized arrhythmias such as AF require less energy to break the circuit (5–10 J internal, 20–100 J external) compared to disorganized rhythms (e.g., ventricular fibrillation, 10–13 J internal, 200–300 J external). The current delivered to the heart is determined by both the energy chosen (J) and the transthoracic impedance [31,32]. The use of bare paddles without a couplant (gel/cream) results in very high transthoracic impedance [31,32]. Impedance may be reduced by strong pressure on the paddles, anteroposterior positioning of the paddles, repetitive shocks, and delivery of the shock during expiration [31,32]. Various types of electrodes may be used for cardioversion, including the traditional handheld electrode paddles and self-adhesive monitor-defibrillator pads. The paddles/pads should be placed in a position that will maximize current flow through the myocardium. The most commonly employed placement is apex–anterior. The anterior electrode should be placed on the right of the upper sternum below the clavicle; the apex electrode should be placed to the left of the nipple, with the center of the electrode in the midaxillary line [33]. In women, placement of paddles on the breast should be avoided, since in this position transthoracic impedance is high. Placement of the apex electrode adjacent to or under the breast is preferable [31]. Synchronization of shocks is extremely important. Properly synchronized shocks rarely, if ever, induce ventricular fibrillation; failure to synchronize shocks on the R wave may lead to inadvertent delivery of a shock in the vulnerable period and is likely to produce ventricular fibrillation [33]. Because some defibrillators default to an unsynchronized mode after each shock, the operator must remember to reenable the synchronizer before each subsequent shock. Shocks to convert atrial fibrillation should begin at energy levels of 100 J, with the energy for subsequent shocks increased in increments of 50–100 J. Atrial flutter is an easier rhythm to convert and shocks should begin at 50 J [33]. For those patients who fail cardioversion, pace termination of AF may be effective. Recently Oral 183 et al. [34] have demonstrated that pretreatment of patients with ibutilide increased the efficacy and decreased the mean energy required for successful transthoracic cardioversion. The role of this approach in ICU patients remains to be determined. In the ICU setting it is highly likely that AF will recur soon after successful cardioversion, as the precipitating conditions still exist. Consequently antiarrhythmic drugs, such as procainamide or amiodarone, administered before or soon after the cardioversion may be useful for prevention of recurrence. Due to the risk of torsades de pointe, all antiarrhythmics should be avoided for at least 4–6 hours in patients who have received ibutilide. Rate Control. Rate control can improve hemodynamics even if the patient remains in AF. Digoxin is commonly used in the treatment of AF in ICU patients. Yet in the critically ill ICU patient, digoxin is probably the most ineffective drug in controlling the ventricular response in AF. Digoxin decreases ventricular response in AF by vagotonic mechanisms [35,36]. In critically ill patients, AF occurs in the setting of high sympathetic tone and frequently with the use of vasopressors and inotropic agents, a situation in which digoxin is likely to be ineffective. Digoxin has been demonstrated to have limited value in the management of patients presenting to hospital with acute AF and in AF after coronary artery bypass graft surgery. Schreck et al. [37] compared the effects of intravenous diltiazem with digoxin for ventricular rate control in the emergent treatment of acute atrial fibrillation and flutter. By 60 minutes, the mean ventricular response had decreased from 150 to 99 beats/min in the diltiazem group and 144–129 beats/min in the digoxin group (p 4 0.01, for differences between groups). Tisdale et al. [38] demonstrated that ventricular rate control occurs more rapidly with intravenous diltiazem than digoxin in AF after coronary artery bypass graft surgery. Other studies have similarly demonstrated poor rate control in patients treated with digoxin [27,39,40]. Furthermore, it is important to emphasize that digoxin does not increase the rate of conversion to sinus rhythm. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group randomized 239 patients (non-ICU) with new onset atrial fibrillation to receive digoxin or placebo [27]. At 16 hours, 46% of the placebo group and 51% of the digoxin group had converted to sinus rhythm (not significant). As digoxin provides poor rate control and has a very narrow therapeutic index, we believe that this drug should be avoided except in ICU patients with poor left ventricular function [40]. Diltiazem appears to be an effective alternative to digoxin for rate control. Ellenbogen et al. [41] 184 Journal of Intensive Care Medicine Vol 15 No 4 July/August 2000 reported that 79 of 84 patients (94%) with acute AF responded to a bolus dose of 20 or 25 mg diltiazem with a greater than 20% reduction in heart rate from baseline, conversion to sinus rhythm, or a heart rate less than 100 beats/min. Hypotension was the most common side effect, occurring in 13% of patients. In an additional report, these authors demonstrated that a 10 to 15 mg/hr continuous infusion of intravenous diltiazem was effective in maintaining rate control [42]. Additional studies have demonstrated a response rate of 93 and 97% [43–45]. Coadministration of calcium is effective in limiting hypotension in diltiazem-treated patients. Esmolol, an ultrashort-acting, nonselective bblocker has also been demonstrated to be effective in controlling the ventricular response in AF [46–48]. Metoprolol (intravenously or orally) is an alternative agent. b-blockers are particularly effective in patients with increased sympathetic tone. These agents may cause hypotension, particularly in unstable ICU patients. Magnesium has been shown to reduce the ventricular response in AF, with a greater effect when combined with digoxin [49,50]. Magnesium may act by increasing the atrio-His interval and atrioventricular nodal refractoriness [51,52]. Adenosine has virtually no role in the acute treatment of AF, given its very short half-life [33]. The agents used for rate control are listed in Table 2. Pharmacologic Cardioversion. A number of antiarrhythmic drugs including procainamide, amiodarone, flecainide, propafenone, sotalol, and ibutilide have been studied in patients with acute atrial fibrillation in a non-ICU setting [17–27]. These trials are listed in Table 3 (inadequately powered, uncontrolled studies after cardiac surgery were excluded from this analysis). The length of observation in many of these studies was short (i.e., 2.5 hours). Only two studies have prospectively investigated the management of AF in ICU patients (Table 1). Procainamide and amiodarone are generally considered to be the antiarrhythmic agents of choice in acutely ill ICU patients [12,14,16]. However, the efficacy of these agents in critically ill ICU patients has never been tested in a randomized controlled study. These agents might best be used in patients with persistent atrial fibrillation in preparation for direct-current cardioversion. The choice between amiodarone and procainamide is determined largely by the drugs side-effect profile and cost, with amiodarone being recommended in patients with severe left ventricular dysfunction. Clemo et al. [16] described 38 critically ill patients with AF who were resistant to conventional heart rate control measures and who were treated with amiodarone. An infusion of amiodarone (over 1 hour) was associated with a significant decrease in heart rate of 37 5 8 beats/min and a significant increase in systolic blood pressure of 24 5 6 mmHg. In the 22 patients with indwelling pulmonary artery catheters, the cardiac index increased over the first 2 hours from 2.4 5 0.3 L/min/m2 to 2.8 5 0.3 L/min/m2 (p < 0.05). In the first 24 hours of the infusion 18 patients (48%) converted to sinus rhythm. Kumar [12] studied the efficacy of intravenous amiodarone in eight patients with severely compromised left ventricular function who developed AF in the ICU. Intravenous amiodarone was effective in seven of the eight patients. Six of the seven patients reverted to sinus rhythm within 30 minutes, with the cardiac index, pulmonary capillary wedge pressure and mean arterial pressure remaining stable during the infusion. Oral and intravenous amiodarone increase atrioventricular nodal refractoriness and intranodal conduction [53–55]. These changes are thought to be mediated by the drug’s calcium channel blocking action and its noncompetitive antiadrenergic effect. Intravenous amiodarone has a minimal effect on the effective refractory periods of atrial, ventricular, and His-Purkinje tissues, and it does not prolong the H-V interval, the QRS interval, or the QTc duration. One hypothesis for this difference is that many of amiodarone’s electrophysiologic effects require long-term administration, which allows greater penetration of the drug and its active metabolite into these tissues [56]. The typical adverse effects seen with chronic use of oral amiodarone (dermato- Table 2. Parenteral Agents Used for Rate Control in AF Agent Loading Dose Maintenance Dose Digoxin 10–15 mg/kg LBW (8–10 mg/kg)* 0.5 mg/kg over 1 minutes 5 mg over 2–4 minutes May repeat every 5 minutes to 15 mg 0.25–0.35 mg/kg over 2 minutes 0.125–0.25 mg/day Esmolol Metoprolol Diltiazem *Patients over 70 years of age and with renal failure. 0.05–0.2 mg/kg/min 5–10 mg every 6 hours 5–15mg/hr Marik and Zaloga: Atrial Fibrillation in the ICU 185 Table 3. Trials of Patients with Recent Onset AF that Developed Outside the ICU Reference Year Number of Patients 17 18 19 20 21 22 23 24 25 26 27 1991 1993 1996 1998 1998 1997 1998 1998 1998 1996 1997 102 80 266 262 127 240 117 156 123 100 239 Drugs Studied Flecainide versus placebo Flecainide versus procainamide Ibutilide versus placebo Ibutilide versus placebo Ibutilide versus procainamide Propafenone versus placebo Propafenone versus procainamide Propafenone versus placebo Propafenone versus digoxin versus placebo Amiodarone versus placebo Digoxin versus placebo logic, ophthalmologic, central nervous system, thyroid) are not observed with intravenous amiodarone [57,58]. Hypotension and bradycardia, the most common adverse events reported with intravenous amiodarone, usually occur during the loading infusion [57]. However, acute hepatotoxicity with liver function test abnormalities has been reported in 9–17% of patients [57,59]. Hepatic dysfunction appears to be short lived and reversible. The early acute hepatitis reported with parenteral amiodarone may be a toxic effect of the carrier vehicle polysorbate 80 [60,61]. Amiodarone has been associated with acute pulmonary toxicity presenting as acute respiratory distress syndrome (ARDS). This syndrome has been reported after thoracic surgery and in infants [62–65]. Donaldson et al. [66] described three patients with ARDS who were treated with amiodarone and had histologic changes of widespread lipoid pneumonia at autopsy. These authors suggest that high oxygen concentrations may be a risk factor for acute amiodarone pulmonary toxicity, while preexisting pathology such as ARDS may mask its development. Amiodarone should therefore be used with caution in this group of patients. It is important to note that amiodarone has been shown to significantly increase plasma concentrations of digoxin by 60–70% within 24 hours of coadministration [67,68]. This interaction is clinically significant and result in signs and symptoms of digitalis toxicity. Procainamide is one of the oldest antiarrhythmic drugs, having been used in 1936 (where it was applied topically to the heart during surgery) [69]. Procainamide is a class IA antiarrhythmic drug with wide antiarrhythmic indications. Intravenously the drug has negative inotropic and hypotensive effects [70]. Nearly half of the administered dose is eliminated unchanged in the urine, with 10–23% of the drug being acetylated to produce N-acetylprocaineamide (NAPA). The elimination of the drug is pro- Length of Observation (Hours) Conversion Success Rate (%) 6 2.5 1.5 1.5 1.5 8 48 2 1 24 16 67 versus 35 92 versus 65 47 versus 2 35 versus 0 58 versus 18 76 versus 37 49 versus 70 70 versus 17 49 versus 32 versus 14 68 versus 60 51 versus 46 longed by both renal and hepatic disease. A close relationship has been found between the plasma concentration and the therapeutic effects of procainamide, with a suggested therapeutic range varying from 4 to 8 mg/ml [69]. QT prolongation and polymorphic ventricular tachycardias (torsades de pointes) may occur with this drug, necessitating close monitoring of both drug levels and the QTc interval on a 12-lead electrocardiogram. Ibutilide fumarate is a new class III intravenous antiarrhythmic agent indicated for the acute termination of atrial fibrillation and flutter [71,72]. It has a short half-life due to extensive tissue distribution and is available only for administration as an intravenous bolus (1 mg dose) [73]. The drug has minimal hemodynamic effects, even in patients with depressed ventricular function [74]. Ibutilide prolongs repolarization in the atria and ventricle by enhancing the inward depolarizing, slow sodium current and by blocking Ikr [71–73]. Ibutilide causes a dose-dependent prolongation of the QT interval, which is maximal at the end of the infusion and returns to baseline within 2–4 hours following infusion [71–73]. Torsade de pointes is the most clinically important adverse effect, being reported in 4.3% of patients [75]. The role of ibutilide in critically ill patients remains to be determined. This drug may have a role in enhancing termination of AF in patients undergoing electrical cardioversion or atrial overdrive pacing [34,76]. Based on the above information we suggest the following approach to the critically ill patient with AF. Hemodynamically unstable patients should be electrically cardioverted followed by an infusion of procainamide or amiodarone (as outlined above). A loading dose of procainamide, amiodarone, or ibutilide followed by repeated attempts at directcurrent cardioversion should be considered after three failed attempts at cardioversion. In ‘‘hemodynamically stable’’ patients with AF, all correctable 186 Journal of Intensive Care Medicine Vol 15 No 4 July/August 2000 factors such as hypokalemia (K < 4 mmol/L), anemia, and hypoxemia should be corrected. Magnesium supplementation (2 g magnesium sulfate in 10 ml dextrose in water over 5 minutes) should be considered except in hypermagnesemic patients (serum magnesium > 2.5 mg/dl). Drugs such as aminophylline and dopamine should be stopped. Pain and anxiety, which increases sympathetic activity, should be treated. We then suggest rate control with diltiazem (5 mg boluses every 5 minutes until rate control is achieved or a maximal dose of Fig 1. Atrial fibrillation/flutter treatment algorithm. 15 mg is administered, followed by an infusion of up to 15 mg/hr). Procainamide or amiodarone should be considered in patients who remain in AF. Amiodarone should be avoided in patients receiving high inspired oxygen concentrations. In patients who remain in AF after 12–24 hours we suggest direct-current electrical cardioversion. This algorithm is outlined in Figure 1. The dosages of the agents used for cardioversion of AF are listed in Table 4. There are no data on the optimal duration of antiarrhythmic therapy. However, as AF is Marik and Zaloga: Atrial Fibrillation in the ICU 187 Table 4. Agents Used for Cardioversion in AF Agent Loading Dose Maintenance Dose Procainamide 5–15mg/kg over 15 minutes (max. 1000 mg) 2–4 mg/min Amiodarone 150 mg over 20–30 minutes Ibutilide 1 mg over 10 minutes (may be repeated in 10 minutes if necessary) 50–150 mg every 8 hours orally 130–300 mg every 8 hours orally 1 mg/min for 6 hours then 0.5 mg/min None Flecainide Propafenone 50–100 mg every 12 hours orally 130–300 mg every 8 hours orally likely to recur if the drug is terminated prematurely, we suggest continuation of the drug (intravenously or orally) until the precipitating factors have either improved or resolved. Atrial Fibrillation and Acute Myocardial Infarction Atrial fibrillation associated with acute myocardial infarction (AMI) most often occurs within the first 24 hours and is usually transient but may recur. The incidence of AF in AMI ranges from 9 to 16% [77–81]. Its etiology includes left ventricular dysfunction, pericarditis, metabolic abnormalities, excess catecholamine release or iatrogenic factors (e.g., use of sympathomimetic drugs) [77,80–82]. The appearance of atrial fibrillation, however, is often a manifestation of extensive left ventricular systolic dysfunction [77,78,82]. Data from the GUSTO-1 study suggest that patients who develop AF frequently have extensive coronary artery disease with poor reperfusion of the infarct-related artery [77]. Patients who develop AF in the setting of AMI are at an increased risk of ischemic strokes [77,78]. Atrial fibrillation in the setting of AMI independently predicts 30-day and 1-year mortality rates [77,78]. If AF causes hemodynamic compromise or ongoing ischemia, direct-current cardioversion should be performed. In the absence of congestive heart failure, bronchospastic disease, or atrioventricular block the most effective means of slowing the ventricular rate in AF is the use of intravenous b-adrenoceptor blocking agents such as atenolol, esmolol, or metoprolol. Rapid digitalization will control the ventricular rate and improve left ventricular function. This method provides a slower response than intravenous b-adrenoceptor blocking agents. Acute digitalization, however, may cause coronary and splanchnic vasoconstriction. Rate slowing may also be achieved with intravenous diltiazem. However, Half-Life (Hours) 3–4 SS (Hours) 12 3–21 (acute) NA 6 NA 16–20 2–10 48–72 24–36 because of their negative inotropic effect and concerns regarding the use of calcium channel blockers in acute AMI, these agents are not recommended as first-line agents. The roles of class I and class III antiarrhythmic agents and electric shock for converting persistent AF are unclear as is the role of antiarrhythmic agents for the prevention of further episodes of AF in patients who convert to sinus rhythm. Anticoagulation with heparin is indicated in patients who remain in AF for more than 24 hours or have recurrent episodes of AF. Anticoagulation According to the most recent guidelines on antithrombotic therapy from the American College of Chest Physicians, anticoagulation is not required for AF of less than 48 hours [83]. This recommendation is supported by the study of Weigner et al. [11] who demonstrated 3 thromboembolic events in 357 patients (1%) with new onset AF (<48 hours) who converted to sinus rhythm. On the other hand, full anticoagulation is required in patients in whom AF has been present for more than 48 hours or its duration is uncertain. In patients planned for pharmacologic cardioversion in whom AF has been present for more than 48 hours and who have not been on chronic anticoagulation, transesophageal echocardiography should be performed to exclude the presence of an atrial thrombus [84]. Conclusion Atrial fibrillation is a common and clinically important arrhythmia in critically ill patients. As this arrhythmia is likely to persist until the underlying medical disorder has resolved, medical management is almost always required. The management of AF includes correction of correctable factors such 188 Journal of Intensive Care Medicine Vol 15 No 4 July/August 2000 as electrolyte abnormalities, elimination of sympathomimetic drugs, rate control, and either pharmacologic or electrical cardioversion. References 1. Artucio H, Pereira M. Cardiac arrhythmias in critically ill patients: epidemiologic study. Crit Care Med 1990;18: 1383–1388 2. Konings KT, Kirchhof CJ, Smeets JR, et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation 1994;89:1665–1680 3. Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:402–405 4. Narayan SM, Cain ME, Smith JM. Atrial fibrillation. Lancet 1997;350:943–950 5. Flaker GC, Fletcher KA, Rothbart RM, et al. Clinical and echocardiographic features of intermittent atrial fibrillation that predict recurrent atrial fibrillation. Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Am J Cardiol 1995; 76:355–358 6. Edwards JD, Kishen R. Significance and management of intractable supraventricular arrhythmias in critically ill patients. Crit Care Med 1986;14:280–282 7. Chazan R, Karwat K, Tyminska K, et al. Cardiac arrhythmias as a result of intravenous infusions of theophylline in patients with airway obstruction. Int J Clin Pharmacol Ther 1995;33:170–175 8. Varriale P, Ramaprasad S. Aminophylline induced atrial fibrillation. Pacing Clin Electrophysiol 1993;16:1953–1955 9. Poukkula A, Korhonen UR, Huikuri H, et al. Theophylline and salbutamol in combination in patients with obstructive pulmonary disease and concurrent heart disease: effect on cardiac arrhythmias. J Intern Med 1989;226:229–234 10. Gelfman DM, Ornato JP, Gonzalez ER. Dopamine-induced increase in atrioventricular conduction in atrial fibrillationflutter. Clin Cardiol 1987;10:671–673 11. Weigner MJ, Caulfield TA, Danias PG, et al. Risk for clinical thromboembolism associated with conversion to sinus rhythm in patients with atrial fibrillation lasting less than 48 hours. Ann Intern Med 1997;126:615–620 12. Kumar A. Intravenous amiodarone for therapy of atrial fibrillation and flutter in critically ill patients with severely depressed left ventricular function. South Med J 1996;89: 779–785 13. Donovan KD, Dobb GJ, Lee KY. Hemodynamic benefit of maintaining atrioventricular synchrony during cardiac pacing in critically ill patients. Crit Care Clin 1991;19: 320–326 14. Chapman MJ, Moran JL, O’Fathartaigh MS, et al. Management of atrial tachyarrhythmias in the critically ill: a comparison of intravenous procainamide and amiodarone. Intensive Care Med 1993;19:48–52 15. Moran JL, Gallagher J, Peake SL, et al. Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med 1995;23:1816–1824 16. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patients with atrial tachyarrhythmias. Am J Cardiol 1998;81: 594–598 17. Donovan KD, Dobb GJ, Coombs LJ, et al. Reversion of recent-onset atrial fibrillation to sinus rhythm by intravenous flecainide. Am J Cardiol 1991;67:137–141 18. Madrid AH, Moro C, Marin-Huerta E, et al. Comparison of flecainide and procainamide in cardioversion of atrial fibrillation. Eur Heart J 1993;14:1127–1131 19. Stambler BS, Wood MA, Ellenbogen KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation 1996;94:1613–1621 20. Abi-Mansour P, Carberry PA, McCowan RJ, et al. Conversion efficacy and safety of repeated doses of ibutilide in patients with atrial flutter and atrial fibrillation. Study Investigators. Am Heart J 1998;136:632–642 21. Volgman AS, Carberry PA, Stambler B, et al. Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol 1998;31:1414–1419 22. Boriani G, Biffi M, Capucci A, et al. Oral propafenone to convert recent-onset atrial fibrillation in patients with and without underlying heart disease. A randomized, controlled trial. Ann Intern Med 1997;126:621–624 23. Mattioli AV, Lucchi GR, Vivoli D, et al. Propafenone versus procainamide for conversion of atrial fibrillation to sinus rhythm. Clin Cardiol 1998;21:763–766 24. Ganau G, Lenzi T. Intravenous propafenone for converting recent onset atrial fibrillation in emergency departments: a randomized placebo-controlled multicenter trial. FAPS Investigators Study Group. J Emerg Med 1998;16:383–387 25. Bianconi L, Mennuni M. Comparison between propafenone and digoxin administered intravenously to patients with acute atrial fibrillation. PAFIT-3 Investigators. The Propafenone in Atrial Fibrillation Italian Trial. Am J Cardiol 1998;82: 584–588 26. Galve E, Rius T, Ballester R, et al. Intravenous amiodarone in the treatment of recent-onset atrial fibrillation: results of a randomized, controlled study. J Am Coll Cardiol 1996;27: 1079–1082 27. Intravenous digoxin in acute atrial fibrillation. Results of a randomized, placebo-controlled multicentre trial in 239 patients. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group. Eur Heart J 1997;18:649–654 28. Kowey PR, Marinchak RA, Rials SJ, et al. Acute treatment of atrial fibrillation. Am J Cardiol 1998;81:C16–C22 29. Anderson JL. Acute treatment of atrial fibrillation and flutter. Am J Cardiol 1996;78(suppl 8A):17–21 30. Pritchett ELC. Management of atrial fibrillation. N Engl J Med 1992;326:1264–1271 31. Kerber RE. Energy requirements for defibrillation. Circulation 1986;74(suppl IV):117–119 32. Sirna SJ, Ferguson DW, Charbonnier F, et al. Factors affecting transthoracic impedance during electrical cardioversion. Am J Cardiol 1988;62:1048–1052 33. American Heart Association’s standards and guidelines for cardiopulmonary resuscitation (CPR) and emergency care. JAMA 1992;268:2184–2241 34. Oral H, Souza JJ, Michaud GF, et al. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med 1999;340:1849–1854 35. Gheorghiade M, Ferguson D. Digoxin. A neurohormonal modulator in heart failure? Circulation 1991;84:2181–2186 36. van Veldhuisen DJ, de Graeff PA, Remme WJ, et al. Value of digoxin in heart failure and sinus rhythm: new features of an old drug? J Am Coll Cardiol 1996;28:813–819 37. Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem Marik and Zaloga: Atrial Fibrillation in the ICU 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. versus intravenous digoxin. Ann Emerg Med 1997;29: 135–140 Tisdale JE, Padhi ID, Goldberg AD, et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J 1998;135:739–747 Jordaens L, Trouerbach J, Calle P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J 1997;18:643–648 Marik PE, Fromm L. A case series of hospitalized patients with elevated digoxin levels. Am J Med 1998;105:110–115 Ellenbogen KA, Dias VC, Cardello FP, et al. Safety and efficacy of intravenous diltiazem in atrial fibrillation or atrial flutter. Am J Cardiol 1995;75:45–49 Ellenbogen KA, Dias VC, Plumb VJ, et al. A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. J Am Coll Cardiol 1991;18: 891–897 Goldenberg IF, Lewis WR, Dias VC, et al. Intravenous diltiazem for the treatment of patients with atrial fibrillation or flutter and moderate to severe congestive heart failure. Am J Cardiol 1994;74:884–889 Salerno DM, Dias VC, Kleiger RE, et al. Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter. The Diltiazem-Atrial Fibrillation/Flutter Study Group. Am J Cardiol 1989;63:1046–1051 Phillips BG, Gandhi AJ, Sanoski CA, et al. Comparison of intravenous diltiazem and verapamil for the acute treatment of atrial fibrillation and atrial flutter. Pharmacotherapy 1997; 17:1238–1245 Shettigar UR, Toole JG, Appunn DO. Combined use of esmolol and digoxin in the acute treatment of atrial fibrillation or flutter. Am Heart J 1993;126:368–374 Platia EV, Michelson EL, Porterfield JK, et al. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol 1989;63:925–929 Byrd RC, Sung RJ, Marks J, et al. Safety and efficacy of esmolol (ASL-8052: an ultrashort-acting beta-adrenergic blocking agent) for control of ventricular rate in supraventricular tachycardias. J Am Coll Cardiol 1984;3:394–399 Hayes JV, Gilman JK, Rubal BJ. Effect of magnesium sulfate on ventricular rate control in atrial fibrillation. Ann Emerg Med 1994;24:61–64 Brodsky MA, Orlov MV, Capparelli EV, et al. Magnesium therapy in new-onset atrial fibrillation. Am J Cardiol 1994; 73:1227–1229 DiCarlo LA, Morady F, de Buitlier M, et al. Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol 1986;7:417–425 Wesley RC, Haines DE, Lerman BB, et al. Effect of intravenous magnesium sulfate on supraventricular tachycardia. Am J Cardiol 1999;63:1129–1131 Nattel S, Talajic M, Quantz M, et al. Frequency-dependent effects of amiodarone on atrioventricular nodal function and slow-channel action potentials: evidence for calcium channel-blocking activity. Circulation 1987;76:442–449 Wellens HJ, Brugada P, Abdollah H, et al. A comparison of the electrophysiologic effects of intravenous and oral amiodarone in the same patient. Circulation 1984;69: 120–124 Ikeda N, Nademanee K, Kannan R, et al. Electrophysiologic effects of amiodarone: experimental and clinical observation relative to serum and tissue drug concentrations. Am Heart J 1984;108:890–898 Desai AD, Sung C, Sung RJ. The role of intravenous amio- 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 189 darone in the management of cardiac arrhythmias. Ann Intern Med 1997;127:294–303 Jafari-Fesharaki M, Scheinman MM. Adverse effects of amiodarone. Pacing Clin Electrophysiol 1998;21:108–120 Kowey PR, Marinchak RA, Rials SJ, et al. Intravenous amiodarone. J Am Coll Cardiol 1997;29:1190–1198 Morelli S, Guido V, De Marzio P, et al. Early hepatitis during intravenous amiodarone administration. Cardiology 1991; 78:291–294 James PR, Hardman SM. Acute hepatitis complicating parenteral amiodarone does not preclude subsequent oral therapy. Heart 1997;77:583–584 Rhodes A, Eastwood JB, Smith SA. Early acute hepatitis with parenteral amiodarone: a toxic effect of the vehicle? Gut 1993;34:565–566 Daniels CJ, Schutte DA, Hammond S, et al. Acute pulmonary toxicity in an infant from intravenous amiodarone. Am J Cardiol 1997;80:1113–1116 Hawthorne HR, Wood MA, Stambler BS, et al. Can amiodarone pulmonary toxicity be predicted in patients undergoing implantable cardioverter defibrillator implantation? Pacing Clin Electrophysiol 1993;16:2241–2249 Van Mieghem W, Coolen L, Malysse I, et al. Amiodarone and the development of ARDS after lung surgery. Chest 1994;105:1642–1645 Mickleborough LL, Maruyama H, Mohamed S, et al. Are patients receiving amiodarone at increased risk for cardiac operations? Ann Thorac Surg 1994;58:622–629 Donaldson L, Grant IS, Naysmith MR, et al. Acute amiodarone-induced lung toxicity. Intensive Care Med 1998;24: 626–630 Lesko LJ. Pharmacokinetic drug interactions with amiodarone. Clin Pharmacokinet 1989;17:130–140 Marcus FI. Drug combinations and interactions with class III agents. J Cardiovasc Pharmacol 1992;20(suppl 2):S70–S74 Ribeiro C, Longo A. Procainamide and disopyramide. Eur Heart J 1987;8(suppl A):11–19 Watanabe H, Chiba S. Cardiovascular effects of quinidine and procainamide on intact dogs and isolated cross-perfused canine atria. J Cardiovasc Pharmacol 1982;4:226–231 Howard PA. Ibutilide: an antiarrhythmic agent for the treatment of atrial fibrillation or flutter. Ann Pharmacother 1999; 33:38–47 Murray KT. Ibutilide. Circulation 1998;97:493–497 Naccarelli GV, Lee KS, Gibson JK, et al. Electrophysiology and pharmacology of ibutilide. Am J Cardiol 1996;78:12–16 Stambler BS, Beckman KJ, Kadish AH, et al. Acute hemodynamic effects of intravenous ibutilide in patients with or without reduced left ventricular function. Am J Cardiol 1997; 80:458–463 Kowey PR, VanderLugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/ flutter. Am J Cardiol 1996;78:46–52 Stambler BS, Wood MA, Ellenbogen KA. Comparative efficacy of intravenous ibutilide versus procainamide for enhancing termination of atrial flutter by atrial overdrive pacing. Am J Cardiol 1996;77:960–966 Crenshaw BS, Ward SR, Granger CB, et al. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol 1997;30: 406–413 Eldar M, Canetti M, Rotstein Z, et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and Thrombolytic Survey Groups. Circulation 1998;97:965–970 190 Journal of Intensive Care Medicine Vol 15 No 4 July/August 2000 79. Howard D, Smith CI, Stewart G. A prospective survey of the incidence of cardiac intoxication with digitalis in patients being admitted to hospital and correlation with serum digoxin levels. Aust N Z J Med 1973;3:279–284 80. Sugiura T, Iwasaka T, Takahashi N, et al. Atrial fibrillation in inferior wall Q-wave acute myocardial infarction. Am J Cardiol 1991;67:1135–1136 81. Sugiura T, Iwasaka T, Takahashi N, et al. Factors associated with atrial fibrillation in Q wave anterior myocardial infarction. Am Heart J 1991;121:1409–1412 82. Sugiura T, Iwasaka T, Ogawa A, et al. Atrial fibrillation in acute myocardial infarction. Am J Cardiol 1985;56:27–29 83. Laupacis A, Albers GW, Dlaen JE, et al. Antithrombotic therapy in atrial fibrillation. Chest 1995;108(suppl):S352–S359 84. Klein AL, Grimm RA, Black IW, et al. Cardioversion guided by transesophageal echocardiography: the ACUTE Pilot Study. A randomized, controlled trial. Assessment of Cardioversion Using Transesophageal Echocardiography. Ann Intern Med 1997;126:200–209