* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download L-Carnitine : Effect on Morbidities and Risk Factors Important to
Coronary artery disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
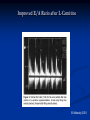
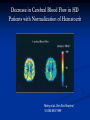
L-Carnitine : Effect on Morbidities and Risk Factors Important to Patients on Dialysis Brian Schreiber,M.D. Assistant Clinical Professor, Department of Medicine, Division of Nephrology Medical College of Wisconsin, Milwaukee, Wisconsin Effect of CHF on Survival in Dialysis Cumulative Survival 1.0 0.8 No CHF 0.6 0.4 0.2 CHF 0 0 12 24 36 48 60 72 Time (Months) Harnett 1995 Five Year Survival Rates H.D with C.H.F Stage IIIA nsc lung Ca COPD, FEV-1 20-30% Stage IIA nsc lung Ca Dukes C2 colon Ca Mean 15% 15% 30% 39% 48% survival metastatic breast cancer is 18 to 24 months. H.D. with CHF mean survival is 32 months. Outline Cardiac Substrate Utilization in normal and pathological disease states Effect of Carnitine on Cardiac Substrate Utilization Carnitine in Ischemia and CHF non-dialysis Carnitine and CHF and LVH in Dialysis patients Cardiac Substrate Preference and Effect of Carnitine Preferred Cardiac Substrate Glucose Intrauterine Left Ventricular Hypertrophy Compensated Cardiomyopathy Pre-dialysis Renal Insufficientcy Fatty Acids extrauterine normals Insulin resistant cardiomyopathy Decompensated cardiomyopathy Dialysis Insulin Resistant Cardiomyopathy Insulin Resistance Independently Predicts Cardiomyopathy Ingelsson E 2005 Insulin resistance predicts Cardiac events in HD Patients Homa IR > 1.6;69% diabetic HD pts 27% non-diabetic HD pts Takaneka 2007 Enhanced Cardiac Glucose Metabolism As compensation for Ischemia Mechanisms For Diabetic Cardiomyopathy Boudina S. 2007 Deleterious Effects of Excess Long Chain Acyl-CoA on Cardiac Myocytes 1) Mitochondrial Permeability Defects 2)Increase Protein Kinase C Beta activity 3)Decreased calcium re-uptake into sarcolemma 4)Decreased glut 4 receptor activity-insulin resistance Carnitine: Metabolic Functions MITOCHONDRIAL CYTOSOL MATRIX Fatty Acids Fatty ACYL Coa Carnitine CPT-1 Carnitine Fatty ACYL Carnitine Fatty ACYL Carnitine CAT Carnitine CPT II GLUCOSE PDH PYRUVATE Fatty ACYL Coa β-oxidation ACETYL Coa Citric Acid Cycle Carnitine Carnitine CAT Acetylcarnitine Acetylcarnitine CAT CO2 Mechanism By Which Carnitine Increases Glucose Oxidation in Hearts Perfused With High Concentrations of Fatty Acids acylcarnitine Carnitine Acyl Fatty Acid Glucose CoA acylcarnitine Carnitine CoA acylCoA CoA pyruvate Acetyl CoA PDH Acetyl CoA Carnitine TCA Cycle Co2 acetylcarnitine acetylcarnitine CoA Carnitine Carnitine In the presence of high levels of carnitine, the increase in the intramitochondrial acetyl CoA levels that is seen in the presence of high concentrations of fatty acids is prevented as a result of the transfer of the acetyl groups from acetyl CoA to acetylcarnitine. The decrease in acetyl CoA levels results in activation of PDH. This increases CO2 production from glucose. PDH, pyruvate dehydrogenase: TCA, tricarboxylic acid cycle, CoA coenzyme A. Fatty Acid Inhibition of Pyruvate Dehydrogenase (PDH) Impact of short- and medium-chain acyl-CoAs (each 250,500, and 1000umol/L, in Tris -HCI, adjusted to pH 7.4) on PDHc activity. All investigated acyl-CoAs inhibited PDHc activity. The inhibitory effect was critically dependent on chain length and number of carboxylic groups. Short-chain monocarboxylic acyl-CoAs revealed the strongest inhibitory effect on PDHc activity. Medium chain and dicaraboxylic acyl-CoAs were less effective inhibitors. Activities are given as percent of control. All data expressed as means ±S.D., experiments were performed intriplicates. Sauer 2008 Ischemia Effects on Acyl-CoA Acetyl CoA 20 15 15 nmol/g 20 Long-chain CoA 10 * 5 25 * 20 0 Cyto Mito nmol/g nmol/g Free CoA * 10 5 * 0 Cyto 15 Mito 10 5 * 0 Cyto Mito Cellular distribution of CoA in the heart. All values are expressed as nmol of wet tissue, and represented as mean ± SEM (n=8). *P<0.01 vs no ischemia. Cyto, cytosolic compartment; Mito, mitochondrial compartment. Kobayashi A, Fujisawa S. J Mol Cell Cardiol. 1994;26:499-508. Carnitine Effect Free CoA Acetyl CoA * 15 * * 10 5 * * 0 20 15 20 * * * * 10 5 Cyto Control Mito 15 10 * * * * 5 * 0 Homo nano mol/g 25 * nano mol/g nmol/g 20 Long-chain CoA 0 Homo Cyto Mito 30 mg/kg carnitine Homo Mito 100 mg/kg carnitine *P<0.01 vs nontreated group (control). Effect of L-carnitine on the cellular distribution of CoA esters in the ischemic heart. All values represent mean ± SEM (n=8). Homo, homogenate (cytosol + mitochondria); Cyto, cytosolic compartment; Mito, mitochondrial compartment. Kobayashi A, Fujisawa S. J Mol Cell Cardiol. 1994;26:499-508. Cyto Carnitine and Cardiac Substrate Metabolism L-Carnitine and Glucose Oxidation in IschemiaReperfusion Aerobic Reperfusion following ischaemia Figure 2 Effect of L-carnitine on glucose oxidation rates in control and diabetic rat heart hearts before and after ischaemia. Values are the means of 7 untreated control, 8 L-carnitine treated control, 8 untreated diabetic and 8 L-carnitine treated diabetic rat hearts. Error bars = SEM. Glucose oxidation rates were determined as described in Methods. *P <0.05 v L-carnitine treated hearts. Broderick 1995 Effects of L-carnitine on Glycolysis & Glucose Oxidation in Isolated Working Hearts Perfused with Fatty Acids Perfusion Conditions Glycolysis (nmol 3H- glucose/g dry wt- min) Glucose Oxidation (nmol 14Cglucose/g dry wtmin) No addition (n=9) 291 0.23 158.4 21.4 Carnitine loaded 4.63 0.46* 454.1 85.3* * Significantly different from those in hearts perfused in the absence of fat Data are the mean S.E.M. of a number of hearts indicated in brackets. Carnitine-loaded hearts were pre-perfused in the working mode for 1 hour with 10 mM carnitine. Glycolysis and glucose oxidation was measured by perfusing hearts with 11 mM (2-3H/U-14C) glucose and 1.2 mM palmitate. Glycolytic rates were determined by measuring ¼ CO2 production. Lopashuk 1992 Lupaschuk 1994 Carnitine and Cardiac Disease: Non-dialysis Selected Studies of Carnitine Treatment in Ischemic Heart Disease in Humans Author # Patients Results Bohles et al, 1987 40 undergoing Pre CABG administration, coronary artery increased, myocardial ATP bypass graft surgery decreased lactate and decrease inotropic medication requirement in post operative period Iliceto et al, 1995 Double-blind placebo controlled 472 with acute MI Decreased LV dilatation and decrease death from CHF in carnitine group Cacciatore et al, 1991 Randomized 200 with stable angina Increased exercise capacity Rizzon et al, 1989 Double-blind placebo controlled 56 with acute MI Significant reduction in ventricular arrhythmias in carnitine treated group Singh 1996 Randomized, double blind, placebo controlled trial lasting 28 days 101 total patients: 51 carnitine, 50 controls Inclusion Criteria: Likely myocardial infarction within 24 hours Patients unable to give consent or with symptoms of MI longer than 24 hours were excluded Intervention Treatment group: L-carnitine 660 mg PO TID Placebo group: aluminum hydroxide 100 mg PO TID Nitrates and aspirin used in 94% of carnitine and 100% of placebo patients Similar use of beta blocker and calcium blocker in both groups(40-50%) Infarct Size Cardiac enzyme activity and electrocardiographic data showing infarct size. Values are means (standard deviations) Carnitine (n = 51) Creatine kinase Size of necrosis (gram equivalents) Maximum latent period before enzyme peak (min) Enzyme peak (IU/l) Area under the curve MB creatine kinase Size of necrosis (gram equivalents) Maximum latent period before enzyme peak (min) Enzyme peak (IU/l) Area under the curve QRS - score Placebo (n = 50) 95.5 (23.6)** 1192.5 (305)* 1.48 (0.78)* 3275 (955)** 116.2 (26.2) 1308 (328) 1.88 (0.92) 4307 (1150) 58.6 (16.6)** 1085 (254)* 1.32 (0.4)* 2790 (715) 7.4 (1.2)** 73.3 (21.5) 1180 (265) 1.55 (0.6) 3110 (680) 10.7 (2.0) * p = <0.05, **p = <0.01. p-value obtained by two-sample t-test comparing carnitine and placebo groups. Cardiac Enzymes Effect of carnitine treatment on cardiac enzymes and lipid peroxides carnitine Enzyme Aspartate transaminase (IU/l) Lactate dehydrogenase (IU/l) Lipid peroxides (nmol/ml) * p <0.05 carnitine No carnitine No carnitine Pre-Rx Post-Rx Pre-Rx Post-Rx 170.0 (15.8) 124.3 (10.5)* 172.2 (17.6) 146.2 (14.2)* 97.5 (10.5) 205.0 (25.6)* 110.3 (12.0) 272.5 (32.6)* 2.8 (0.25) 1.8 (0.22)* 2.7 (0.23) 2.6 (0.12)* Complications Complications at 28 days of follow-up. Values are numbers (percentages) Carnitine Placebo Complications (n = 51) (n = 50) Angina pectoris 9 (17.6)* 18 (36.0) NYHA Class III and IV heart failure Left ventricular enlargement 4 (7.8) 7 (14.0) 8 (15.6) 11 (22.0) 12 (23.4)* 18 (36.0) 6 (11.7) 11 (22.0) 1 (1.9) 3 (6.0) 7 (13.7)* 14 (28.0) Hypotension (systolic <90 mmHg) 1 (1.9) 3 (6.0) Cardiac end points: total cardiac deaths 4 (7.8) 6 (12.0) nonfatal reinfarction 4 (7.8)* 7 (14.0) total cardiac events 8 (15.6)* 13 (26.0) Total cases with poor left ventricular function Ventricular ectopics (>8/min) Ventricular ectopics (>3 consecutively) Total arrhythmias Relative risk (95) CL) 0.49 (0.98, 0.24) 0.56 (1.86, 0.17) 0.71 (1.61, 0.31) 0.65 (1.24, 0.35) 0.54 (1.32, 0.24) 0.31 (2.79, 0.03) 0.49 1.11, 0.21) 0.31 (2.79, 0.03) 0.65 (1.88, 0.22) 0.56 (1.86, 0.17) 0.60 (1.62, 0.27) * p = <0.05; p-value significant by Z score test for proportions by comparison of carnitine group with placebo. CL = confidence limits; NYHA = New York Heart Association. Levocarnitine Treatment in CHF in Humans-Nondialysis Author Ferrari, et al double blind, randomized placebo controlled Kobayashi, et al intent to treat analysis Rizos, et al, 2000 double blind, randomized placebo controlled Patients 574 NYHA Class II-III EF<40% 40 80 NYHA Class III-IV Results Carnitine group: exercise tolerance improved, nonimprovement in mortality or hospital admission Improvement to lower NYHA class, in 55% of patients receiving carnitine Carnitine group had lower mortality rate (P<0.04). Statistically significant improvement in VO2 Max, Weber Class, cardiac output and lower rate of arrhythmias Carnitine & Muscle Apoptosis in CHF OD 405 nm (% of control) 250 *# 200 150 # * 100 50 ne F ca rn i ti CH CH F + Co nt ro l 0 *P < 0.01; #P < 0.05 Vescovo 2002 Carnitine and Cardiac Disease: Dialysis Cardiac Substrate Uptake in Predialysis CKD,Normal Patients, and CHF Mean Myocardial Glucose Uptake Normal=27.6 µmol/min/100g CKD Non-dialysis=68.9µmol/min/100g Dilated Cardiomyopathy=12.3µmol/min/1006 Fink 2010 Increased NEFA in Hemodialysis Patients 1250 [NEFA] (µmol.L -1) 1000 750 500 250 0 healthy control CKD, non-dialysis PD HD post treatment HD prior to treatment Gillett M Saudi Med J 2004 25(11)1611-1616 Abnormality of Skeletal Fatty Acid Metabolism in Dialysis Fatty acid oxidation control = 1487 ± 267 dpm/mg Fatty acid oxidation hemodialysis patients = 638 ± 285 dpm/mg (p < 0.003 control vs HD) (Savica 1983) Myocardial Fatty Acid Metabolism in Uremic Patients Measured With BMIPP Scintigraphy H/M Ratio WOR % Chronic HD-Carn 1.91+/-.19 * 17.%+/-6.0 * Chronic HD+carn 1.89+/-2.0 * 21.9+/-6.6 ** Non HD control 1.52+/-.24 22.8+/-4.2 * p<.05 vs.non HD **p<.001 vs.-Carn Sakurabayashi Am J Nephrol 1999:19:480-484 L-carnitine Plasma Concentrations in ESRD patients During the First Year of Dialysis Treatment 60 Plasma Conc (nmol/ml) 55 50 normal range 45 40 35 30 25 20 0 0.2 0.4 0.6 Time on dialysis (yr) 0.8 1 Evans AM, et al. Kidney Int. 2004;66:1527-1534. Decreased Plasma Free Carnitine Levels in Hemodialysis Patients Value prior to Hemodialysis (maximum value for HD patients) 19.2 ± 6.5 μmol/liter (Sakurabayashi 1999) (a/f=.87) 24.8 ± 7.9 μmol/liter (Suzuki 1982) 21.5 ± 7 μmol/liter (van Es 1992) (a/f=.98) 28 ± 6.0 μmol/liter (Bellinghieri 1983) 24.4 ± 8.528.2 ± 6.5 μmol/liter (Sakurabayashi 1999) 19.5 ± 5.6 μmol/liter (Evans 2000) (a/f=.77) 25.9 μmol/liter (Lennon 1986) (a/f=.96) 32.4 μmol/liter (Rossle 1985) 30.4 μmol/liter (Savica 1983) (Normal control value 40 50 μmol/liter) (a/f<.4) Reason for Dialysis Related Carnitine deficiency 70% of plasma carnitine removed with each dialysis decreased dietary intake decreased synthetic capacity Mean Plasma Carnitine Concentrations 350 Carnitine m mol/L 300 TC treatment TC placebo FC treatment FC placebo SCAC treatment SCAC placebo LCAC treatment LCAC placebo 250 200 150 100 50 0 Week 0 Week 12 Week 24 TC – total carnitine, FC – free carnitine, SCAC – short chain acylcarnitine, LCAC- long chain acylcarnitine; Radioenzymatic assay technique, between treatment and placebo groups - p<0.01 Mean C8-C14 Acylcarnitine 0.8 0.7 0.6 mmole/L 0.5 0.4 0.3 0.2 0.1 0 0 12 C8 4methyl of C9 24 C10 Baseline to 12 weeks and baseline to 24 weeks, p<0.01 Except C14 = p<0.05, n=13 C12 C14 Mean C16-C18 acylcarnitine 0.25 mmol/L 0.2 0.15 0.1 0.05 0 0 12 C16 C16:2 24 C18 Baseline to 12 weeks and baseline to 24 weeks, p<0.01, except C18:1 = p<0.05, n=13 C18:2 Mean C16-C18 acylcarnitine 0.25 mmol/L 0.2 0.15 0.1 0.05 0 0 12 C16 C16:2 24 C18 Baseline to 12 weeks and baseline to 24 weeks, p<0.01, except C18:1 = p<0.05, n=13 C18:2 Acetylcarnitine (mmol/L) Changes in Acetylcarnitine 80 70 60 50 40 30 20 10 0 Baseline 12 Weeks 24 Weeks Baseline to 12 weeks and baseline to 24 weeks, p<0.01, n=13 Decrease in CRP in Patients with High Baseline 5 CRP (mg/dl) 4 3 2 1 0 0 1 2 3 4 5 6 Follow-up (months) Placebo Carnitine Placebo Carnitine Savica V Journal of Renal Nutrition 2005 15(2) 225230 L-carnitine and Markers of Free Radical Metabolism in HD Parameter baseline 3 mos 6 mos 9 mos SOD u/g/hgb 1.044 1.034 1.043 983 GSH perox 38.4 40.8 45.8* 38.4‡ GSHmmol/l 1.65 1.90* 2.23** 1.67‡ Antiox cap 1.65 1.67 2.06* 1.52‡ Sel mcg/l 54 38.2* 49.5 59.5 MDA µmol/l 4.18 3.48* 3.07*** 2.82 Tot Prot g/l 65.6 73.5* 71.8** 66.7† Alb g/l 34.8 40.8 46.0* 43.2 Vesela 2001 Carnitine and Plasma Glucose Disappearance in HD Patients Placebo (n=6) L-Carnitine (n=7) Before After P Before After P Fasting glucose (mmol/L) 5.1 ± 0.2 4.94 ± 0.4 NS 5.19 ± 0.2 4.84 ± 0.1 NS C-peptide (µg/L) 4.90 ± 0.9 5.01 ± 0.1 NS 5.98 ± 0.9 7.35 ± 2.3 NS Insulin (µmol/L) 36.9 ± 7.6 38.1 ± 8.2 NS 40.60 ± 5.4 32.80 ± 4.0 NS 3.30 ± .4 3.37 ± 0.4 NS 2.99 ± 0.3 3.54 ± 0.2 <0.03 Kitt (%/min)* *Kitt: rate constant for plasma glucose disappearance Gunal A.Journal of Nephrology Vol 12 no.1 1999 38-40 Systolic Dysfunction Development of CHF 1.0 0.8 Normal 0.6 Concentric LVH 0.4 0.2 Systolic Dysfunction 0 0 12 24 36 48 60 LV Dilation 72 Time (Months) Harnett 1995 van Es et al. 1992 Three months Symptomatic (n=7) Asymptomatic (n=9) 1 g levocarnitine intravenously after each dialysis session van Es A, et al. In Guarnieri G, Panzetta G, Toigo G (eds.): Metabolic and Nutritional Abnormalities in Kidney Disease. Contrib Nephrol. Basal, Karger 1992; 98:28-35. Effect of L-Carnitine Therapy on Left Ventricular Ejection Fraction Symptomatic patients (n=7) FC (µmol) TC, (µmol/l) 1 2 5.3 10.2 16.7 30.5 31.7 33.4 15 26 35 35 3 4 5 6 7 19.4 21.4 22.4 22.5 31.6 40.3 46.2 41.5 41.2 59.3 48.1 46.3 54.0 54.6 53.3 14 46 17 49 46 27 60 25 44 66 30.4+16.0 41.7+15.9 19.0+8.7 Carnitine EF before (% free) L-carnitine, (%) 39.4+13.2 45.9+9.7 EF after 3 mos. of L-carnitine, (%) A.van ES et al Contrib Nephrol, 1992 Romagnoli et al. 2001 Changes in Left Ventricular Ejection Fraction During Carnitine Therapy 45 40 35 LVEF% 30 25 20 15 10 5 0 Baseline 2 Mo. 4 Mo. 6 Mo. 8 Mo. Romagnoli GF, Noso A, Carraro G, et al. Beneficial Effects of L-Carnitine in Dialysis Patients with Impaired Left Ventricular Function: an Observational Study. Curr Med Res Opin 18(3):1-4, 2002 LVH as a Pivotal Intermediary in Dialysis related Cardiomyopathy Cardiac Ischemia Cardiac Dilatation LVH Congestive Heart Restrictive Cardiomyopathy Failure Change in LVH and Event Free Survival Zoccali C 2004 Causes of LVH in ESRD Patients Anemia Hypertension Volume overload Increased growth factor levels Abnormal glucose and fatty acid metabolism Hyperinsulinemia Promotes Left Ventricular Hypertrophy Sharma N 2007 Carnitine and LVH-Sakurabayashi 2008 10 patients given 10mg/kg L carnitine 3x/week post dialysis for one year 10 untreated controls Primary endpoints-Echocardiographic change in indices of LVH Carnitine and LVH-Dialysis Number M/F Age (years) Duration of HD (years) Primary renal disease Chronic glomerulonephritis Diabetes mellitus Antihypertensives Calcium antagonists ACE inhibitors ARB β-blockers r-HuEPO Carnitine No Carnitine 10 9/1 45.7±13.7 12.2±4.5 10 9/1 46.0±7.8 12.5±4.2 9 1 6 6 5 2 2 7 8 2 8 6 5 2 1 6 Data are mean±SD. HD, hemodialysis; Antihypertensives, number of patients taking antihypertensive medication; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; rHuEPO, number of patients taking recombinant human erythropoietin medication. Sakurabayashi T 2008 Circ J 72:926-931 Change in Cardiac Dimensions and Mass with L-Carnitine in ESRD Beginning LVEDD (cm) LVESD (cm) IVS (cm) LVPW (cm) IVS + LVPW (cm) LVFS (%) LVM (g) LVMI (g/m2) End Carnitine No Carnitine Carnitine No Carnitine 5.03±0.65 3.35±0.43 1.04±0.17 1.15±0.22 2.19±0.35 33.2±5.3 243.0±34.7 151.8±21.2 5.29±0.84 3.39±0.72 1.40±0.17 1.05±0.15 2.09±0.31 36.2±5.0 247.8±40.2 153.3±28.2 5.00±0.37 3.31±0.39 1.00±0.11 1.02±0.12* 2.02±0.22 33.9±4.7 217.1±36.8** 134.0±16.0** 5.28±0.90 3.38±0.80 1.14±0.34 1.08±0.17 2.22±0.48 36.6±6.2 270.6±65.9 167.1±43.1 *p<0.05, **p<0.01 within the group. Data are mean±SD. LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; IVS, interventricular septal end-diastolic thickness; LVPW, left ventricular posterior wall end-diastolic thickness; LVFS, left ventricular fractional shortening; LVM, left ventricular mass; LVMI, left ventricular mass index. Sakurabayashi T 2008 Circ J 72:926-931 Carnitine and Diastolic Dysfunction in Pediatric Hemodialysis 24 Children on chronic hemodialysis treated with L-carnitine 50mg/kg orally after each hemodialysis for two months 24 healthy children of similar age and socioeconomic status as controls Age range 8-19 years Mean number of months on hemodialysis=19 El-Metwally et al 2003 Carnitine and Diastolic Function ESRD Reduced E/A ratio pre-carnitine El-Metwally 2003 Improved E/A Ratio after L-Carnitine El-Metwally 2003 Importance of Red Cell Deformability in Microcirculation Baskurt O Meiselman H (2003). Blood rheology and hemomdynamics. Seminars in Thrombosis and Hemostasis 29 (5) 435-450. Decrease in Cerebral Blood Flow in HD Patients with Normalization of Hematocrit Metry et al J Am Soc Nephrol 10:854-863 1999 Deformability of RBC In Hemodialysis Patients Before and After the Dialysis Session, as Well as in Normal Controls Before HD n Before HD After HD (after LC) 1 17.99 27.92 10.4 2 3 4 5 6 7 8 9 10 11 12 13 14 15 15.68 11.90 15.78 10.08 11.80 15.78 12.34 15.27 19.71 13.19 20.58 17.77 13.88 13.12 16.44 21.25 24.11 19.29 22.81 34.67 19.73 16.33 20.01 13.49 22.63 25.65 25.00 16.71 14.53 11.02 14.62 10.03 11.02 10.00 11.90 15.44 10.29 9.50 10.40 11.76 6.60 14.70 Mean ± SD 15.0 ± 3.1 21.7 ± 5.4 11.5 ± 2.4 (before HO vs normal controls p < 0.00001), (before HD vs after HD p < 0.00001), (before HD vs before HD after 3 months LC supplementation, p < 0.004), (before HD after 3 months LC supplementation vs normal control, p < 0.02). Sotirakopoulos, Nikolaos Renal Failure, 22(1), 73-80(2000) Trovato (2) Bellinguieri (11) Fagher (7-9) (95 % Cl: 0.02;1.00) overall effect p=0.01 Htest 0.20 Labonia (17) Patrikarea (18) Megri (19) Caruso (20) Altmann (23) (95 % Cl: -1.46;-0.05) p=0.01 Htest 0.20 Kletzmayr (22) overall effect -3.5 -2.5 -1.5 -0.5 0.5 1.5 The effect of L-carnitine on anemia control (top; fixed model analysis) and erythropoietin dose reduction (bottom; random model analysis). Open boxes indicate the effect of each individual trial, and the closed boxes the overall effect of treatment. The P value is given for overall treatment effect and is significant for values ≤ 0.01. Heterogeneity of treatment effect among trials is present if H test value is < 0.10. Decrease in Hospitalization With Levocarnitine Therapy-Kazmi et al 2005 Relative Risk of Hospitalization 1 0.9 Non cardiac 0.8 Cardiac History 0.7 0.6 0.5 0.4 0.3 0.2 2 -1 to -9 -9 to -6 -6 to -3 -3 to 0 0 to + 3 + 3 to + 6 + 6 to + 9 + 9 to + 12 + 12 to + 15 + 15 to + 18 + 18 to + 21 + 21 to + 24 Months before and after initiation of carnitine therapy Kazmi WH, et al. Am J Nephrol. 2005;25:106-115. USRDS Data 1998-2003 Weinhandl 2007 Peritoneal Dialysis Free carnitine level below normal but not as low as hemodialysis Elevated acyl/free carnitine ratio indicative of fatty acid metabolic abnormality Serum free carnitine falls as effluent volumes and adequacy increase Limited clinical data for improvement in muscle biochemistry and EPO resistance Constantin –Teodosiu 1996 Plasma free carnitine concentration 28.5µmol/liter,significantly lower than control Daily loss of free carnitine in CAPD significantly greater than healthy controls Total/free carnitine ratio greater in plasma than controls “These ratio differences suggests that an alteration in acyl group metabolism is occurring in CAPD patients Constantin-Teodosiu et al Kidney Int.1996 49 (1):158-162 Sotirakopoulos 2002 ● 12 adult patients on CAPD > 6 months 2 grams L-carnitine per os x 3 months Hct ↑ 35.4 →38.1 (p<.03) Hb ↑ 11.0 →11.9 (p<.01) rHuEPO dose ↓3833 →1292+/- (p<.01) RBC IR(index de regidite)↓16.6→13.0 (p<.03) Sotirakopoulos N et al Renal Failure 2002 24(4) 505-510 Summary Cardiac substrate metabolism is an important predictor of cardiac physiology Prior to starting dialysis,CKD patients are in a state where increased cardiac glucose metabolism is a partial compensation for cardiac disease Dialysis patients, almost always carnitine deficient,have excess myocardial unmetabolized fatty acids The excess fatty acids have major harmful effects on myocardial cells including inhibition of glucose metabolism Administration of L-carnitine to dialysis patients has been associated with improved cardiac function in CHF and a reduction of LVH