* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Skin and Soft Tissue: Diabetic Foot Infections
Gastroenteritis wikipedia , lookup
Sarcocystis wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Cryptosporidiosis wikipedia , lookup
Trichinosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Marburg virus disease wikipedia , lookup
Onchocerciasis wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Clostridium difficile infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hepatitis B wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Methicillin-resistant Staphylococcus aureus wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Staphylococcus aureus wikipedia , lookup
Neonatal infection wikipedia , lookup
Anaerobic infection wikipedia , lookup
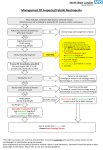
Skin Skinand andSoft SoftTissue: Tissue: Diabetic Foot Infections Diabetic Foot Infections SEVERITY OF INFECTION Mild • Only skin and subcutaneous tissue involvement AND • Erythema > 0.5 cm and ≤ 2 cm around ulcer • Perform incision and drainage as necessary Moderate** • Deeper tissue involvement OR • Erythema > 2.0 cm around ulcer AND • No systemic signs of infection • Perform incision and drainage as necessary SUSPECTED ORGANISMS RECOMMENDED EMPIRICAL TREATMENT DURATION MSSA Streptococcus spp. Oral Amoxicillin/clavulanate 875 mg PO Q12H OR Cephalexin 500 mg PO Q6H OR Dicloxacillin 250 – 500 mg PO Q6H MRSA Doxycycline 100 mg PO Q12H OR SMX/TMP 2 DS tablets PO Q12H (Does not cover Group A Strep) Oral OR Initially Parenteral 1–3 weeks Ampicillin-sulbactam 1.5–3 gm IV Q6H OR Ceftriaxone 1 gm IV Q24H MSSA Streptococcus spp. Enterobacteriaceae Obligate anaerobes 1–2 weeks Penicillin Allergy: MRSA Pseudomonas aeruginosa Ciprofloxacin 500 mg PO Q12H AND Clindamycin 300 mg PO Q6H OR Ceftriaxone 1 gm IV Q24H Linezolid 600 mg IV/PO Q12H† (Requires ID Consult) OR Daptomycin 6 mg/kg IV Q24H† (Requires ID Consult) OR Vancomycin 15 mg/kg IV* Piperacillin-tazobactam 3.375 gm IV Q4H DS= Double Strength; H= hour(s); IV= intravenous; MRSA= methicillin resistant S. aureus; MSSA= methicillin sensitive S. aureus; PO= by mouth; Q= every; SMX-TMP= sulfamethoxazole/trimethoprim; spp= species † Restricted Antibiotic – refer to Table of Contents for Guidelines for Restricted Antimicrobials * Refer to Table of Contents for section on Vancomycin Dosing and Monitoring in Adult Patients ** Consult Infectious Diseases and Podiatry NOTE: Dosing based on normal renal function. Refer to Table of Contents for section on Antimicrobial Dosing for Adult Patients Based on Renal Function PAGE 24 Skin and Soft Tissue: Diabetic Foot Infections Skin Skinand andSoft SoftTissue: Tissue: Diabetic Foot Infections Diabetic Foot Infections R E SEVERITY OF INFECTION SUSPECTED ORGANISMS ECOMMENDED MPIRICAL DURATION TREATMENT RECOMMENDED EMPIRICAL SUSPECTED ORGANISMS Initially Parenteral DURATION MSSA/MRSA TREATMENT P. aeruginosa Vancomycin 15 mg/kg IV* MSSA/MRSA Initially Parenteral Streptococcus spp. AND** P. aeruginosa 2–4 weeks Enterobacteriaceae Vancomycin 15 IV mg/kg Cefepime 2 gm Q8HIV* + Streptococcus spp. Obligate anaerobes AND** metronidazole 500 mg IV Q6H 2–4 weeks Enterobacteriaceae Cefepime 2 gm IV Q8H + OR Obligate anaerobes metronidazole 500 mg IV Q6H Piperacillin-tazobactam OR 3.375 gm IV Q4H Piperacillin-tazobactam 3.375 gm IV Q4H Bone OR Joint Involvement‡ SevereS**EVERITY OF INFECTION • Same as moderate ** Severe AND •• Same as moderate Systemic signs of infection AND present • Systemic signs of infection Systemic Inflammatory presentSyndrome (SIRS) Response Systemic Criteria ≥2Inflammatory of the following: Response Syndrome (SIRS) • Temperature Criteria ≥2 of the <96.8°F following: OR >100.4°F •• Temperature P > 90 BPM <96.8°F SourceOR removed: 2-5 days ‡ Joint Involvement Bone • OR RR >>100.4°F 20 BPM •• PPaCO > 90 BPM < 32 mmHg Source removed but 2 removed: 2-5 residual days tissue infection: •• RR > 20 BPM cells/mm³ WBC < 4000 1-3 weeks • PaCO Source removed but residual tissue infection: 2 < 32 mmHg OR >12,000 cells/mm³ Source removed but residual bone infection: •• WBC 4000 cells/mm³ 1-3 weeks ≥ 10%< immature (band) 4-6 weeks >12,000 cells/mm³ OR forms Source removed but residual bone infection: •• ≥Perform 10% immature (band) incision and Source not removed: ≥3 months 4-6 weeks forms drainage as necessary • Perform incision and Source not removed: ≥3 months BPM= beats or breaths per minute; H= hour(s); IV= intravenous; MRSA= methicillin resistant S. aureus; MSSA= methicillin drainage as necessary sensitive S. aureus; P= pulse; PaCO2= partial pressure of carbon dioxide; Q= every; RR= respiratory rate; SIRS= Systemic Inflammatory Syndrome; spp= species; white blood cellmethicillin resistant S. aureus; MSSA= methicillin BPM= beats orResponse breaths per minute; H= hour(s); IV=WBC= intravenous; MRSA= sensitive S. aureus; P= pulse; PaCO2= partial pressure of carbon dioxide; Q= every; RR= respiratory rate; SIRS= Systemic † Restricted Antibiotic – refer to Table of Contents for Guidelines for Restricted Antimicrobials Inflammatory Response Syndrome; spp= species; WBC= white blood cell * Refer to Table of Contents for section on Vancomycin Dosing and Monitoring in Adult Patients Consult Infectious and Podiatry †**Restricted AntibioticDiseases – refer to Table of Contents for Guidelines for Restricted Antimicrobials Discuss Infectious Podiatry, and Vascular *‡ Refer toplan Tablewith of Contents forDiseases, section on Vancomycin Dosing and Monitoring in Adult Patients ** Consult Infectious Diseases and Podiatry NOTE: Dosing based on normal renal function. Refer to Table of Contents for section on Antimicrobial Dosing for Adult ‡ Discuss plan with Infectious Diseases, Podiatry, and Vascular Patients Based on Renal Function NOTE: Dosing based on normal renal function. Refer to Table of Contents for section on Antimicrobial Dosing for Adult Patients Based on Renal Function References: 1. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis 2012;54(12):e132-73. References: 2. insert]. York, 2015.EJ, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the 1. Flagyl Lipsky[package BA, Berendt AR, New Cornia PB, NY: Pile Pfizer; JC, Peters Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis 2012;54(12):e132-73. 2. Flagyl [package insert]. New York, NY: Pfizer; 2015. PAGE 25