* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy Resources
Energy storage wikipedia , lookup
Conservation of energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Multi-junction solar cell wikipedia , lookup
World energy consumption wikipedia , lookup
Open energy system models wikipedia , lookup
Solar cell research wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
100% renewable energy wikipedia , lookup
Flow battery wikipedia , lookup
Renewable energy debate wikipedia , lookup
Alternative energy wikipedia , lookup
Negawatt power wikipedia , lookup
Shockley–Queisser limit wikipedia , lookup
Energy policy of Australia wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Microbial fuel cell wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
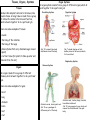
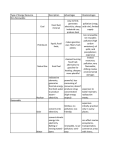
Year 7 Science Revision The topics that you will tested on on Monday 2nd December are: • Energy resources • Acids and alkalis • Cells, tissues and organ systems Revision is very important if you want to achieve your best. Our memory works by constantly using information. Revising means we are using the information. Useful Websites: 1. The BBC bitesize website has lots of information, activities and quizzes for you to revise from. Remember to choose the topics you have covered in year 7! http://www.bbc.co.uk/bitesize/ks3/science/ 2. Download short revision clips to your computer or mobile phone from the following website: Be sure you use the clips relevant to your year! http://www.collinseducation.com/Downloads/Pages/SeriesDownloads.aspx?Level1=Primary,Secondary&SeriesTitle=Collins%20 Revision&rt=Videos 3. Test yourself using Doc Brown’s quizzes. Be sure you test yourself on the test relevant to your year! http://www.docbrown.info/ks3science.htm Energy Resources Energy Resources Energy Resources and the environment Non- renewable energy Also know as fossil fuels. Examples of fossil fuels: • coal • oil • gas They are non-renewable because they have taken millions of year to make and we can’t make them in our lifetime (they will run out). Geothermal Energy Uses the heat energy in the Earth to generate electricity + no pollution - Found in very few places in the world Wave Energy Uses kinetic energy in waves to generate electricity + no pollution - Needs lots of machines to get enough energy, look ugly Tidal Energy Uses the gravitational potential energy in tides to generate electricity + reliable as always two tides a day - Costs a lot to build a local dam, could cause flooding Biomass Uses the chemical energy in living things to generate electricity + no special equipment needed so can used in poorer countries - Large areas of land are needed to grow enough trees Renewable energy resources Examples of renewable energy resources are: • solar power • wind power • tidal power • wave power • hydroelectric power • geothermal power • biomass They are renewable because they will not run out like fossil fuels will in our lifetime. Hydroelectric Energy Uses flowing water (kinetic energy) to generate electricity + no pollution - Costs a lot of money to build a dam Wind Energy Uses the wind (kinetic energy) to generate electricity + no pollution, quite cheap and easy to build - Some people think they are ugly, only works if windy! Solar Energy Uses the sun (heat energy) to generate electricity or heat up water + no pollution, renewable (the sun won’t run out any time soon!) - expensive, only works if sunny! Nuclear Energy Uses the chemical energy in metals to generate electricity + no harmful gases produced - non-renewable (uranium will run out), produces dangerous radioactive waste Recycling, Reusing, Reducing Recycling, reusing, reducing helps the environment because it means new objects don’t have to be made using energy resources. This can reduce pollution. Examples of what you could do are: using reusing plastic bags, turning lights off when you don’t need them, recycling aluminium cans. Acids and Alkalis Acids Hazard Symbols Alkalis • Acids contain the element hydrogen (H). • Alkalis contain a hydroxide (OH oxygen and hydrogen). • Examples of acids in the home are vinegar, oranges and grapefruits. • Examples of alkalis in the home are soaps, oven cleaners and washing powders. • Examples of acids in the science laboratory are hydrochloric acid and sulphuric acid. Indicators Indictors are used to say whether liquids or solutions are acidic, neutral or alkaline • Examples of alkalis in the science laboratory are sodium hydroxide, potassium hydroxide. Litmus paper Litmus paper is an indicator. If in a neutral solution it’s colour is unchanged, it turns red in an acidic solution and blue in an alkaline solution. pH scale Acids and alkalis are corrosive and have to be used carefully. Scientists use hazard symbols to help know about the dangers of chemicals. i Highly flammable – easily catches fire h Harmful – e.g. when swallowed, breathed in Radiation – possibly causing DNA damage Universal indicator can be used to measure how strong or weak an acid or alkali is (this means it gives more information than Litmus paper). Universal indicator is a mixture of several dyes extracted from plants. The overall colour the indicator solution is compared with the range of colours in the pH scale (above). A neutral solution has a pH of 7. An acidic solution has a pH of 1 to 6 (strong acid = 1 to 3, weak acid = 4 to 6). An alkaline solution has a pH of 8 to 14 (weak alkali = 8 to 10, strong alkali = 11 to 14). Corrosive – attacks and destroys living tissues Toxic - Can cause death Environmental hazard – substance that is dangerous to the environment Irritant– can cause reddening and blistering of the skin Oxidising – provides oxygen to make other substances burn Explosive – substance that can explode Neutralisation Reactions between acids and alkalis are called neutralisation reactions. We use neutralisation reactions to help us: • Indigestion tablets to reduce excess stomach acid • Farmers add lime (alkali) to soil to neutralise excess acidity from acid rain. •Toothpaste (alkali) neutralises acid that builds up on our teeth. Cells Animal and plant cells Specialised Cells Cell Function Adaptation Absorbs and carries oxygen around the body Large cell membrane surface area Carries messages around the body Long and thin Develops into an embryo Large and contains lots of cytoplasm Fertilise the egg Has a long tail allowing it to move Red blood cell Nerve cell Functions of animal and plant cell parts Part Function (job) Found in animal cells Found in plant cells Cell membrane Controls what passes into and out of the cell Nucleus Contain the genetic material and control the cell activities Cytoplasm Jelly-like liquid where important chemical reaction take place Egg cell Sperm cell Absorb water Cell wall Made of cellulose and supports the cell, helping it keep its shape Vacuole Contains cell sap Chloroplasts Contain chlorophyll which traps light for use in photosynthesis (where plants make their own food) Large cell membrane surface area Root hair cell Site of photosynthesis Palisade cell Contains lot of chloroplasts Tissues, Organs, Systems Tissues Animal cells and plant cells can form tissues, like muscle tissue. A living tissue is made from a group of cells with a similar structure and function, which all work together to do a particular job. Organ Systems An organ system is made from a group of different organs, which all work together to do a particular job. Circulatory System Digestive System Here are some examples of tissues: heart • muscle • the lining of the intestine capillaries • the lining of the lungs • phloem (tubes that carry dissolved sugar around a plant) Job: To transport blood and substances around the body. • root hair tissue (for plants to take up water and minerals from the soil) Organs Job: To break food we eat into smaller pieces so that we can absorb it into our blood. Respiratory System Nervous System An organ is made from a group of different tissues, which all work together to do a particular job. Here are some examples of organs: • heart • lung • stomach • brain • leaf • root Contains: nerves, brain, spinal cord. Job: To carry messages to difference part of the body Contains: nose, trachea, lungs, bronchus, bronchioles and alveoli Job: To bring oxygen into your body, and remove the carbon dioxide from your body.