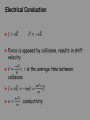* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 14. Elementary Particles
Survey
Document related concepts
Transcript
Elementary Particles (last bit); Start Review for Final Start Review for Final Exam Final Exam: Tuesday Dec 15th, 8am to 10am in Physics 203 Steven Weinberg (1933 - ) Particles and Antiparticles Particles and Antiparticles Of course “strangeness” and “charmness” also change for antiparticles containing strange and charm quarks Basic properties in changing from particle to antiparticle: Same mass (m) Same spin (J) Opposite charge (q) and (-q) May also have other quantum numbers that change Review General guidelines: 4 problems – exam is 2 hours No explicit problems on Special Relativity From the hydrogen atom on is fair game; expect a foundational question on quantum mechanics (particle in the box, harmonic oscillator, periodic potential etc.) Emphasis will be on subjects covered since last exam: Solids, electronic properties of metals and semiconductors, and elementary particles Conductors, Insulators, Semiconductors NaCl is an insulator, with a band gap of 2 eV, which is much larger than the thermal energy atT=300K Therefore, only a tiny fraction of electrons are in the conduction band Conductors, Insulators, Semiconductors Silicon and germanium have band gaps of 1 eV and 0.7 eV, respectively. At room temperature, a small fraction of the electrons are in the conduction band. Si and Ge are intrinsic semiconductors Band Diagram: Intrinsic Semiconductor T>0 Conduction band (Partially Filled) EC EF EV Valence band (Partially Empty) At T = 0, lower valence band is filled with electrons and upper conduction band is empty, leading to zero conductivity. Fermi energy EF is at midpoint of small energy gap (<1 eV) between conduction and valence bands. Donor Dopant in a Semiconductor For group IV Si, add a group V element to “donate” an electron and make n-type Si (more negative electrons!). “Extra” electron is weakly bound, with donor energy level ED just below conduction band EC. Dopant electrons easily promoted to conduction band, increasing electrical conductivity by increasing carrier density n. Fermi level EF moves up towards EC. EC EF EV n-type Si ED Egap~ 1 eV Band Diagram: Acceptor Dopant in Semiconductor For Si, add a group III element to “accept” an electron and make p-type Si (more positive “holes”). “Missing” electron results in an extra “hole”, with an acceptor energy level EA just above the valence band EV. Holes easily formed in valence band, greatly increasing the electrical conductivity. Fermi level EF moves down towards EV. EC EF EV EA p-type Si pn Junction: Band Diagram Due to diffusion, electrons move from n to p-side and holes from p to n-side. Causes depletion zone at junction where immobile charged ion cores remain. Results in a built-in electric field (103 to 105 V/cm), which opposes further diffusion. Note: EF levels are aligned across pn junction under equilibrium. EC EF EV n-type electrons pn regions “touch” & free carriers move EF holes p-type pn regions in equilibrium EC EF EV –– – +– – –– + + + + + +–– –– – + ++ ++ Depletion Zone Forward Bias and Reverse Bias Forward Bias : Connect positive of the positive end to positive of supply…negative of the junction to negative of supply Reverse Bias: Connect positive of the junction to negative of supply…negative of junction to positive of supply. PN Junction: Under Bias • Forward Bias: negative voltage on n-side promotes diffusion of electrons by decreasing built-in junction potential higher current. • Reverse Bias: positive voltage on n-side inhibits diffusion of electrons by increasing built-in junction potential lower current. Equilibrium p-type n-type e– Forward Bias p-type n-type Reverse Bias –V e– Majority Carriers p-type n-type e– Minority Carriers +V pn Junction: IV Characteristics Current-Voltage Relationship I I o [e eV / kT 1] Forward Bias: current exponentially increases. Reverse Bias: low leakage current equal to ~Io. Ability of pn junction to pass current in only one direction is known as “rectifying” behavior. Forward Bias Reverse Bias Manifestly not a resistor: V=IR Not Ohm’s law Heat Capacity of Electron Gas By definition, the heat capacity (at constant volume) of the electron gas is given by dU CV dT where U is the total energy of the gas. For a gas of N electrons, each with average energy <E>, the total energy is given by UN E Heat Capacity of Electron Gas Therefore, the total energy can be written as kT 3 U NEF NkT 5 EF where = p2/4 dU p T CV Nk dT 2 TF 2 Total Heat Capacity Electrons + Lattice Electrical Conduction Resistivity resistivity as a function of n and FE ma E m 1 e e 2 J ne vd ne (a ) n ne Temperature dependence • Metal: Resistance increases with Temperature. Why? Temp , n same (same # conduction electrons)