* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ph507lecnote06
Hawking radiation wikipedia , lookup
Dyson sphere wikipedia , lookup
Corvus (constellation) wikipedia , lookup
H II region wikipedia , lookup
Observational astronomy wikipedia , lookup
Future of an expanding universe wikipedia , lookup
Stellar evolution wikipedia , lookup
Star formation wikipedia , lookup
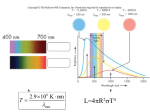
PH507 Astrophysics Professor Glenn White 1 The planet inj the news: Lecture 9: Radiation processes Almost all astronomical information from beyond the Solar System comes to us from some form of electromagnetic radiation (EMR). We can now detect and study EMR over a range of wavelength or, equivalently, photon energy, covering a range of at least 1016- from short wavelength, high photon energy gamma rays to long wavelength low energy radio photons. Out of all this vast range of wavelengths, our eyes are sensitive to a tiny slice of wavelengthsroughly from 4500 to 6500 Å. The range of wavelengths our eyes are sensitive to is called the visible wavelength range. We will define a wavelength region reaching somewhat shorter (to about 3200 Å) to somewhat longer (about 10,000 Å) than the visible as the optical part of the spectrum. (Note: Physicists measure optical wavelengths in nanometers (nm). Astronomers tend to use _Angstroms. 1 Å = 10-10 m = 0.1 nm. Thus, a physicist would say the optical region extends from 320 to 1000 nm.) All EMR comes in discrete lumps called photons. A photon has a definite energy and frequency or wavelength. The relation between photon energy (Eph) and photon frequency is given by: Eph = h or, since c = PH507 Astrophysics Professor Glenn White E ph 2 hc where h is Planck’s constant and is the wavelength, and c is the speed of light. The energy of visible photons is around a few eV (electron volts). (An electron volt is a non- metric unit of energy that is a good size for measuring energies associated with changes of electron levels in atoms, and also for measuring energy of visible light photons. 1 eV = 1.602 x 10-19 Joules.) In purely astronomical terms, the optical portion of the spectrum is important because most stars and galaxies emit a significant fraction of their energy in this part of the spectrum. (This is not true for objects significantly colder than stars e.g. planets, interstellar dust and molecular clouds, which emit in the infrared or at longer wavelengths - or significantly hotter- e.g. ionised gas clouds, neutron stars, which emit in the ultraviolet and x-ray regions of the spectrum. Another reason the optical region is important is that many molecules and atoms have electronic transitions in the optical wavelength region. Blackbody Radiation Where then does a thermal continuous spectrum come from? Such a continuous spectrum comes from a blackbody whose spectrum depends only upon the absolute temperature. A blackbody is so named because it absorbs all electromagnetic energy incident upon it - it is completely black. To be in perfect thermal equilibrium, however, such a body must radiate energy at exactly the same rate that it absorbs energy; otherwise, the body will heat up or cool down (its temperature will change). Ideally, a blackbody is a perfectly insulated enclosure within which radiation has come into thermal equilibrium with the walls of the enclosure. Practically, blackbody radiation may be sampled by observing the enclosure through a tiny pinhole in one of the walls. The gases in the interior of a star are opaque (highly absorbent) to all radiation (otherwise, we would see the stellar interior at some wavelength!); hence, the radiation there is blackbody in character. We sample this radiation as it slowly leaks from the surface of the star - to a rough approximation, the continuum radiation from some stars is blackbody in nature. We will define the regions of the Electromagnetic Spectrum to have wavelengthds as follows: Gamma-rays: < 0.1Å, highest frequency, shortest wavelength, highest energy. X-Rays: 0.1Å -- 100Å Ultraviolet light: 100Å -- 3000Å Visible light: 3000Å -- 10000Å = 1µm (micrometer or micron) PH507 Astrophysics Professor Glenn White 3 Infrared Light: 1µm -- 1mm Radio waves: >1mm, lowest frequency, longest wavelength, lowest energy. Planck’s Radiation Law After Maxwell's theory of electromagnetism appeared in 1864, many attempts were made to understand blackbody radiation theoretically. None succeeded until, in 1900, Max K. E. L. Planck (1858-1947) postulated that electromagnetic energy can propagate only in discrete quanta, or photons, each of energy E = hv. He then derived the spectral intensity relationship, or Planck blackbody radiation law: 2h 3 1 I( )d 2 h c kT e 1 where I(v)dv is the intensity (J/m2 . s . sr) of radiation from a blackbody at temperature T in the frequency range between v and v + dv, h is Planck's constant, c is the speed of light, and k is Boltzmann's constant. Note the exponential in the denominator. Because the frequency v and wavelength of electromagnetic radiation are related by v = c, we may also express Planck's formula in terms of the intensity emitted per unit wavelength interval: This is illustrated for several values of T: PH507 Astrophysics Professor Glenn White 4 Note that both I() and I(v) increase as the blackbody temperature increases - the blackbody becomes brighter. This effect is easily interpreted when we note that I(v)∆v is directly proportional to the number of photons emitted per second near the energy hv. The Planck function is special enough so that its given its own symbol, B() or B(v), for intensity. Wien’s Law A blackbody emits at a peak intensity that shifts to shorter wavelengths as its temperature increases. PH507 Astrophysics Professor Glenn White 5 Wilhelm Wien (1864-1928) expressed the wavelength at which the maximum intensity of blackbody radiation is emitted - the peak (that wavelength for which dI()/d = 0) of the Planck curve (found from taking the first derivative of Planck's law) - by Wien's displacement law: max = 2.898 x 10-3 / T where max is in metres when T is in Kelvin. Note that because maxT = constant, increasing one proportionally decreases the other. For example, the continuum spectrum from our Sun is approximately blackbody, peaking at max ≈ 500 nm. Therefore, the surface temperature is near 5800 K. The Law of Stefan and Boltzmann The area under the Planck curve (integrating the Planck function) represents the total energy flux, F (W/m2), emitted by a blackbody when we sum over all wavelengths and solid angles: PH507 Astrophysics Professor Glenn White 6 where = 5.669 x 10-8 W/m2 . K4. The strong temperature dependence of this formula was first deduced from thermodynamics in 1879 by Josef Stefan (18351893) and was derived from statistical mechanics in 1884 by Boltzmann. Therefore we call the expression the Stefan-Boltzmann law. The brightness of a blackbody increases as the fourth power of its temperature. If we approximate a star by a blackbody, the total energy output per unit time of the star (its power or luminosity in watts) is just L = 4R2T4 since the surface area of a sphere of radius R is 4R2 To summarise: A blackbody radiator has a number of special characteristics. One, a blackbody emits some energy at all wavelengths. Two, a hotter blackbody emits more energy per unit area and time at all wavelengths than does a cooler one. Three, a hotter blackbody emits a greater proportion of its radiation at shorter wavelengths than does a cooler one. Four, the amount of radiation emitted per second by a unit surface area of a blackbody depends on the fourth power of its temperature. Stellar Material Our Sun is the only star for which I( has been accurately observed. Indeed, Ibol is related to the solar constant: the total solar radiative flux received at the Earth’s orbit outside our atmosphere (1370 W/m2). The solar luminosity L (3.86 x 1026 W) is calculated from the solar constant in the following manner. Using the inverse-square law, we find the radiative flux at the Sun’s surface R. Then Lis just times this flux. The solar energy distribution curve may be approximated by a Planck blackbody curve at the effective temperature Teff, defined as the temperature of a blackbody that would emit the same total energy as an emitting body, such as the Sun or a star. Then the Stefan-Boltzmann law implies L = 4π R2 T4eff where is the Stefan-Boltzmann constant. Stellar Atmospheres J s-1 PH507 Astrophysics Professor Glenn White 7 The spectral energy distribution of starlight is determined in a star’s atmosphere, the region from which radiation can freely escape. To understand stellar spectra, we first discuss a model stellar atmosphere and investigate the characteristics that determine the spectral features. Physical Characteristics The stellar photosphere, a thin, gaseous layer, shields the stellar interior from view. The photosphere is thin relative to the stellar radius, and so we regard it as a uniform shell of gas. The physical properties of this shell may be approximately specified by the average values of its pressure P, temperature T, and chemical composition µ (chemical abundances). We also assume that the gas obeys the perfect-gas law: P =nkT where k is Boltzmann’s constant. This relationship is also known as Boyle’s Law. An important result that follows from it is that the kinetic energy of a particle, or assemblage of particles, is given by the relationship; KE 3 kT 2 Thus temperature is just a measure of the kinetic energy of a gas, or an assemblage of particles. This equation applies equally well to a star as a whole, as to a single particle, and later we will look at the comparison between a star’s kinetic and gravitational (potential) energies. The kinetic energy is also a measure of the velocity that atoms or molecules are moving about at - the hotter they are, the faster they move. Thus, for a cloud of gas surrounding a hot star of temperature T = 15,000 K, which consists of hydrogen atoms (mass = 1.67 10-27 kg); 3 1 kT KE mv2 2 2 v 3kT 19 km s 1 50,000 mph m The particle number density is related to both the mass density (kg/m3) and the composition (or mean molecular weight) µ by the following definition of µ: 1 mH n PH507 Astrophysics Professor Glenn White 8 where mH = 1.67 x 10-27 kg is the mass of a hydrogen atom. For a star of pure atomic hydrogen, µ = 1. If the hydrogen is completely ionised, µ = 1/2 because electrons and protons (hydrogen nuclei) are equal in number and electrons are far less massive than protons. In general, stellar interior gases are ionised and 1 3 1 2X Y Z 4 2 where X is the mass fraction of hydrogen, Y is that of helium, and Z is that of all heavier elements. The mass fraction is the percentage by mass of one species relative to the total. Thus, for a pure hydrogen star (X=1.0, Y = 0.0, Z = 0.0), ~ 0.5, and for a white dwarf star (X = 0.0, Y = 1.0, Z = 0.0) ~ 1.33. Temperatures The continuous spectrum, or continuum, from a star may be approximated by the Planck blackbody spectral-energy distribution. For a given star, the continuum defines a colour temperature by fitting the appropriate Planck curve. We can also define the temperature from Wien’s displacement law: maxT = 2.898 x 10-3 m . K which states that the peak intensity of the Planck curve occurs at a wavelength max that varies inversely with the Planck temperature T. The value of max then defines a temperature. Also note here that the hotter a star is, the greater will be its luminous flux (in W/m2), in accordance with the Stefan-Boltzmann law: F = T4 where = 5.67 x 10-8 W/m2 . K4. Then the relation L = 4πR2T4eff defines the effective temperature of the photosphere. A word of caution: the effective temperature of a star is usually not identical to its excitation (Boltzmann eqn) or ionisation temperature (Saha eqn) because spectralline formation redistributes radiation from the continuum. This effect is called line blanketing and becomes important when the numbers and strengths of spectral lines are large. PH507 Astrophysics Professor Glenn White 9 When spectral features are not numerous, we can detect the continuum between them and obtain a reasonably accurate value for the star’s effective surface temperature. The line blanketing alters the atmosphere’s blackbody character. Spectrophotometry The goal of the observational astronomer to to make measurements of the EMR from celestial objects with as much detail, or the finest resolution, possible. There are of course different types of detail that we want to observe. These include angular detail, wavelength detail, and time detail. The perfect astronomical observing system would tell us the amount of radiation, as a function of wavelength, from the entire sky in arbitrarily small angular slices. Such a system does not exist! We are always limited in angular and wavelength coverage, and limited in resolution in angle and wavelength. If we want good information about the wavelength distribution of EMR from an object (spectroscopy or spectrophotometry) we have to give up angular detail. If we want good angular resolution over a wide area of sky (imaging) we usually have to give up wavelength resolution or coverage. The ideal goal of spectrophotometry is to obtain the spectral energy distribution (SED) of celestial objects, or how the energy from the object is distributed in wavelength. We want to measure the amount of energy received by an observer outside the Earth's atmosphere, per second, per unit area, per unit wavelength or frequency interval. Units of spectral flux (in cgs) look like: f = ergs s-1 cm-2 Å -1 if we measure per unit wavelength interval, or f = ergs s-1 cm-2 Hz -1 PH507 Astrophysics Professor Glenn White 10 (pronounced f nu if we measure per unit frequency interval. Classifying Stellar Spectra Observations A single stellar spectrum is produced when starlight is focused by a telescope onto a spectrometer or spectrograph, where it is dispersed (spread out) in wavelength and recorded photographically or electronically. If the star is bright, we may obtain a high-dispersion spectrum, that is, a few mÅ per millimetre on the spectrogram, because there is enough radiation to be spread broadly and thinly. At high dispersion, a wealth of detail appears in the spectrum, but the method is slow (only one stellar spectrum at a time) and limited to fairly bright stars. Dispersion is the key to unlocking the information in starlight. The Spectral-Line Sequence At first glance, the spectra of different stars seem to bear no relationship to one another. In 1863, however, Angelo Secchi found that he could crudely order the spectra and define different spectral types. Alternative ordering schemes appeared in the ensuing years, but the system developed at the Harvard Observatory by Annie J. Cannon and her colleagues was internationally adopted in 1910. This sequence, the Harvard spectral classification system, is still used today. (About 400,000 stars were classified by Cannon and published in various volumes of the Henry Draper Catalogue, 1910-1924, and its Extension, 1949. At first, the Harvard scheme was based upon the strengths of the hydrogen Balmer absorption lines in stellar spectra, and the spectral ordering was alphabetical (A through to P). Some letters were eventually dropped, and the ordering was rearranged to correspond to a sequence of decreasing temperatures (see the effects of the Boltzmann and Saha equations): OBAFGKMRNS. Stars nearer the beginning of the spectral sequence (closer to O) are sometimes called early-type stars, and those closer to the M end are referred to as late-type. Each spectral type is divided into ten parts from 0 (early) to 9 (late); for example, . . . F8 F9 G0 G1 G2 . . . G9 K0 . . . . In this scheme, our Sun is spectral type G2. In 1922, the International Astronomical Union (IAU) adopted the Harvard system (with some modifications) as the international standard. Many mnemonics have been devised to help students retain the spectral sequence. A variation of the traditional one is “Oh, Be a Fine Girl, Kiss Me Right Now, Smack.” The next Figure shows exemplary stellar spectra arranged in order; note how the conspicuous spectral features strengthen and diminish in a characteristic way through the spectral types. PH507 Astrophysics Professor Glenn White 11 Comparison of spectra observed for seven different stars having a range of surface temperatures. The hottest stars, at the top, show lines of helium and multiply-ionised heavy elements. In the coolest stars, at the bottom, helium lines are not seen, but lines of neutral atoms and molecules are plentiful. At intermediate temperatures, hydrogen lines are strongest. The actual compositions of all seven stars are about the same. PH507 Astrophysics Professor Glenn White 12 The Temperature Sequence The spectral sequence is a temperature sequence, but we must carefully qualify this statement. There are many different kinds of temperatures and many ways to determine them. Theoretically, the temperature should correlate with spectral type and so with the star’s colour. From the spectra of intermediate-type stars (A to K), we find that the (continuum) colour temperature does so, but difficulties occur at both ends of the sequence. For O and B stars, the continuum peaks in the far ultraviolet, where it is undetectable by ground-based observations. Through satellite observations in the far ultraviolet, we are beginning to understand the ultraviolet spectra of O and B stars. For the cool M stars, not only does the Planck curve peak in the infrared, but numerous molecular bands also blanket the spectra of these low-temperature stars. PH507 Astrophysics Professor Glenn White 13 PH507 Astrophysics Professor Glenn White 14 When the strengths of various spectral features are plotted against excitationionisation (or Boltzmann-Saha) temperature; the spectral sequence does correlate with this temperature as seen below; In practice, we measure a star’s colour index, CI = B - V, to determine the effective stellar temperature. If the stellar continuum is Planckian and contains no spectral lines, this procedure clearly gives a unique temperature, but observational uncertainties and physical effects do lead to problems: (a) for the very hot O and B stars, CI varies slowly with Teff and small uncertainties in its value lead to very large uncertainties in T; (b) for the very cool M stars, CI is large and positive, but these faint stars have not been adequately observed and so CI is not well determined for them; (c) any instrumental deficiencies, calibration errors, or unknown blanketing in the B or V bands affect the value of CI - and thus the deduced T. Hence, it is best to define the CI versus T relation observationally. SPECTROSCOPY • Last year discussed stellar spectra and classification on an empirical basis: Spectral sequence O B A F G K M Temperature ~40,000 K ----> 2500 K Classification based on relative line strengths of He, H, Ca, metal, molecular lines. • We will now look a little deeper at stellar spectra and what they tell us about stellar atmospheres. PH507 Astrophysics Professor Glenn White 15 Radiative Transfer Equation • Imagine a beam of radiation of intensity I passing through a layer of gas: Power passing into volume Area dA E = I d dA d Power passing out of volume E + dE where I = intensity into solid angle element d path length ds NB in all these equations subscripts can be replaced by In the volume of gas there is: ABSORPTION - Power is reduced by amount dE = - E ds = - I d dA d ds where is the ABSORPTION COEFFICIENT or OPACITY = the cross-section for absorption of radiation of wavelength (frequency ) per unit mass of gas. Units of are m2 kg-1 The quantity is the fraction of power in a beam of radiation of wavelength absorbed by unit depth of gas. It has units of m-1. (NB in many texts is simply given the symbol in the equations given here - beware!) EMISSION - Power is increased by amount dE = j d dA d ds (1) where j = EMISSION COEFFICIENT = amount of energy emitted per second per unit mass per unit wavelength into unit solid angle. Units of j (j) are W kg-1 µm-1 sr-1 (W kg-1 Hz-1 sr-1) or m s-3 sr-1 (NB power production per unit volume per unit wavelength into unit solid angle is =j More confusion is possible here, since is also the symbol used for total power output of a gas, units are W kg-1, - Beware!) So total change in power is dE = dI d dA d = - I d dA d ds + j d dA d ds which reduces to dI = - I ds + j ds (2) PH507 Astrophysics dI Professor Glenn White 16 = - I + j ds (3) This is a form of the radiative transfer equation in the plane parallel case. Optical depth • Take a volume of gas which only absorbs radiation (j = 0) at : dI = - I ds For a depth of gas s, the fractional change in intensity is given by I (s) s dI = - ds I I (0) 0 ln ( Integrating ==> I (s) I (0) s ) = - ds 0 s - I (s) = I (0) e ==> ds 0 We define Optical depth s ds So (4) - I (s) = I (0) e (5) • Intensity is reduced to 1/e (=1/2.718 = 0.37 ) of its original value if optical depth = 1. • Optical depth is not a physical depth. A large optical depth can occur in a short physical distance if the absorption coefficient is large, or a large physical distance if is small. Full Radiative Transfer Equation again dI = - I + j ds divide by PH507 Astrophysics dI Professor Glenn White j = -I + ds 17 dI d = -I + S (6) As ds --> 0, is constant over ds. This is the RADIATIVE TRANSFER EQUATION in the plane parallel case. Define: j S = or j = S where and S is the SOURCE FUNCTION. Radiative transfer in a blackbody • Remember definition of a blackbody as a perfect absorber and emitter of radiation. Matter and radiation are in THERMODYNAMIC EQUILIBRIUM, ie gross properties do not change with time. Therefore a beam of radiation in a blackbody is constant: dI = 0 = - I + j ds ==> 0 = (I - S), i.e. I = S. but for a blackbody I = B 2 B = 2hc the PLANCK FUNCTION 3 1 hc/kT (e from definition of source function, j = S - 1) B = 2h 2 c 1 hkT (e - 1) Summary: in complete thermodynamic equilibrium the source function equals the Planck function, i.e. j = B • In studies of stellar atmospheres we make the assumption of LOCAL THERMODYNAMIC EQUILIBRIUM (LTE), i.e. thermodynamic equilibrium for each particular layer of a star. • Note that if incoming radiation at a particular wavelength (e.g. in a spectral line) enters a blackbody gas it is absorbed, but emission is distributed over all wavelengths according to the Planck function. All information about the original energy distribution of the radiation is lost. This is what happens in interior layers of a star where the density is high and photons of any wavelength are absorbed in a very short distance. Such a gas is said to be optically thick (see below). Emission and Absorption lines (7) (K PH507 Astrophysics Professor Glenn White 18 • the absorption coefficient describes the efficiency of absorption of material in the volume of gas. In a low density gas, photons can generally pass through without interaction with atoms unless they have an energy corresponding to a particular transition (electron energy level transition, or vibrational/rotational state transition in molecules). At this particular energy/frequency/wavelength the absorption coefficient is large. • Let's imagine the volume of gas shown earlier with both absorption and emission: I I (0) path length s dI d = S - I Multiply both sides by e and re-arrange dI ==> d e + I e = S e d ==> d (I e ) = S e integrate over whole volume, i.e. from 0 to s, or 0 to I e ==> = 0 S e 0 assuming S = constant along path I e - I(0) = S e - S ==> ==> I I(0) e- + radiation left over from light entering box. = S (1 - e- ) light from radiation emitted in the box. (8) >> 1: OPTICALLY THICK CASE If >> 1, then e- --> 0, and eqn (8) becomes I = S In LTE S = B, the Planck function. • Case 1 (9) PH507 Astrophysics Professor Glenn White 19 So for an optically thick gas, the emergent spectrum is the Planck function, independent of composition or input intensity distribution. True for stellar photosphere (the visible "surface" of a star). • Case 2 << 1 OPTICALLY THIN CASE If << 1, then e- ≈ 1 - (first two terms of Taylor series expansion) eqn (8) becomes I = ==>I = I(0) + ( S - I(0) ) I(0) (1 - ) + S (1 - 1 + ) • If I(0) = 0 : no radiation entering the box (from direction of interest): From eqn (8) I = S (= B in LTE) Since = ∫ , then I = s S If is large (true at wavelength of spectral lines) then I is large, we see EMISSION LINES. This happens for example in gaseous nebulae or the solar corona when the sun is eclipsed. • If I(0) ≠ 0 , let's examine eqn (8) I = I(0) + ( S - I(0) ) If S > I(0) then right hand term is +ve when is large (ie is large) we see higher intensity than I(0) ==> EMISSION LINES ON BACKGROUND INTENSITY. If S < I(0) then right hand term is -ve when is large (ie is large) we see lower intensity than I(0) ==> ABSORPTION LINES ON BACKGROUND INTENSITY. For stars we see absorption lines. This means I(0) > S, i.e. (intensity from deeper layers) > (source function for the top layers Assuming LTE (S = B) the source function increases as temperature increases: I(0) = B(Tdeep layer) > S = B(Touter layer). Therefore temperature must be increasing as we go into the star for absorption lines to be observed. • To summarise - We see CONTINUUM RADIATION for an optically thick gas (= PLANCK FUNCTION assuming LTE). - We see EMISSION LINES for an optically thin gas. - We see ABSORPTION LINES + CONTINUUM for an optically thick gas overlaid by optically thin gas with temperature decreasing outwards. - We see EMISSION LINES + CONTINUUM for an optically thick gas overlaid by an optically thin gas with temperature increasing outwards. (10) PH507 Astrophysics Professor Glenn White 20