* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Proteolysis of the platelet surface: dissociation of shape change from
Survey
Document related concepts
Transcript
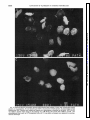
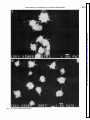
Proteolysis of the platelet surface: dissociation of shape change from aggregation ELIZABETH KORNECKI, AND ROBERT H. LENOX YIGAL H. EHRLICH, DAVID H. HARDWICK, Neuroscience Research Unit, Departments of Psychiatry and Biochemistry, University of Vermont College of Medicine, Burlington, Vermont 05405 Stimulation of intact platelets by ADP resultsin a shapechange followed by aggregationin the presenceof fibrinogen. ADP was found to induce a shapechangein chymotrypsin-treated platelets that was similar in extent and initial velocity to that of intact (untreated) platelets. Scanning-electron microscopyverified an ADP-induced shape change in chymotrypsin-treated platelets. This shapechange could be completely blocked by stimulators of platelet adenylate cyclase (forskolin, prostaglandin E1, and prostacyclin). On the other hand, the aggregation of chymotrypsin-treated platelets by fibrinogen was not dependent on the presenceof ADP and could not be blocked by forskolin, prostaglandin E1, or prostacyclin, even though the levels of cyclic AMP (CAMP) formed in chymotrypsin-treated platelets were comparable to levels that completely inhibited the ADP-induced aggregation of intact platelets. This lack of inhibition of platelet aggregationwasnot due to degradation of the adenylate cyclase or prostaglandin receptors, sincechymotrypsin-treated platelets were found to have a functional adenylate cyclase system that could be stimulated by forskolin, prostaglandin E1, and prostacyclin and inhibited by ADP and epinephrine, similar to that of intact platelets. These results provide direct evidence that CAMP does not interact with fibrinogen binding sites once they have becomepermanently exposedon the surface of platelets. Pretreatment of platelets with chymotrypsin therefore appearsto be a useful tool that allowsfor the dissociationof platelet shapechangefrom aggregation, without inhibiting either response. chymotrypsin treatment of human platelets; cyclic AMP; shape change;fibrinogen-induced aggregation MEMBRANES contain fibrinogen receptors consisting of glycoproteins IIb and IIIa that are responsible for platelet aggregation. Platelets that are not activated do not bind radiolabeled fibrinogen; such fibrinogen receptors remain latent, allowing platelets to circulate as single disks. However, when platelets are stimulated by ADP (1, 12, 16, 25), thrombin (9, 26), epinephrine (1, 27), or prostaglandin endoperoxides (2, 20), the platelets change shape from disk to sphere, extend long pseudopods, and expose specific fibrinogen binding sites on the cell surface. The exposure of these sites on adjoining platelets results in fibrinogen binding and platelet aggrePLATELET H550 0363-6135/86 gation. The aggregation and shape change of platelets can be inhibited by prostaglandin E1, forskolin, and prostacyclin I2 (8), agents that raise levels of intracellular cyclic AMP (CAMP) by stimulating the adenylate cyclase of platelets (7, 8, 10, 18, 19). Hawiger et al. (9) and Graber and Hawiger (5) have shown that the binding of radiolabeled fibrinogen to ADP or thrombin-activated platelets can be inhibited by these agents. Several laboratories have demonstrated that fibrinogen binding sites become exposed on the platelet surface by pretreating platelets with proteolytic enzymes such as chymotrypsin or pronase (6,13,23). These platelets have been shown to specifically bind fibrinogen and to aggregate spontaneously on the addition of fibrinogen. In contrast to intact (untreated) platelets, proteolytically treated platelets do not require the presence of ADP or any other platelet agonist for fibrinogen binding and aggregation. The treatment of platelets with chymotrypsin has been used as a tool for the identification of fibrinogen binding domains of the platelet fibrinogen receptor (13, 17). Furthermore, proteolysis by chymotrypsin provides a platelet model for the investigation of pathological conditions in which chymotrypsin and other enzymes are found in high concentrations in the circulation. The effects of pancreatic enzymes on platelet activation have been implicated in the development of systematic complications of pancreatitis (28). Proteolysis of platelet surface glycoproteins in vivo has been associated with liver cirrhosis (24). Egbring et al. (3) measured high concentrations of granulocyte elastase in the plasma of patients with septicemia or acute leukemia, which may be associated with thrombocytopenia. Damage of fibrinogen receptors resulting in thrombocytopenia and platelet dysfunction, concurrent with increased concentrations of neutrophil elastase, were found to occur during simulated extracorporeal circulation (2 1, 35). The purpose of the present study was to determine whether treatment of the platelet surface with chymotrypsin has differential effects on platelet shape change, aggregation, the platelet adenylate cyclase system, and on the function of several receptors coupled to the system. The results demonstrate that treatment of the platelet surface with an extracellular protease provides a most useful tool for investigating the mechanisms of receptormediated platelet functions. $1.50 Copyright 0 1986 the American Physiological Society Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 KORNECKI, ELIZABETH, YIGAL H. EHRLICH, DAVID H. HARDWICK, AND ROBERT H. LENOX. Proteolysis of the platelet surface: dissociation of shape change from aggregation. Am. J. Physiol. 250 (Heart Circ. Physiol. 19): H550-H557, 1986.- ACTIVATION MATERIALS AND OF PLATELETS METHODS SURFACE PROTEOLYSIS H551 Suspensions containing 10’ platelets/ml were preincubated with chymotrypsin (500 or 1,000 pg) for 1 h at 37OC. Control (untreated) platelet suspensions were incubated for 1 h at 37°C in the absence of chymotrypsin. Both types of platelet suspensions were washed by centrifugation for 10 min at 1,600 g and resuspended in Tyrode-albumin containing buffer at a concentration of 2 x log/ml. Four hundred fifty microliters of each suspension were aliquoted into an aggregometer cuvette under stirring conditions at 37OC. Aliquots of ADP (50 ~1; 50 PM, final concn) or distilled H20 were added to both intact and chymotrypsin-treated platelets. After 1 min, the platelets were fixed with 1.5% glutaraldehyde (final concn) buffered with 0.1 M phosphate buffer, pH 7.4. After fixation, coverslips coated with platelets were dehydrated in alcohol, critical point dried from Freon mounted on carbon stubs, vacuum coated with goldpalladium, and examined in a JEOL JSM-35C scanning electron microscope. RESULTS Human platelets that have been pretreated with various concentrations of chymotrypsin were aggregated directly upon addition of fibrinogen (Fig. 1). In contrast to intact (untreated) platelets, ADP was not necessary for aggregation to occur. Moreover, the fibrinogen-induced aggregation of chymotrypsin-treated platelets was not accompanied by a shape change. The fibrinogeninduced aggregation was dependent on the concentration of chymotrypsin used during the preincubation period. Maximal aggregation to fibrinogen was observed with platelets preincubated with 1,000 pg of chymotrypsin, whereas minimal fibrinogen-induced aggregation occurred with platelets preincubated with less than 500 pg of chymotrypsin. To determine the changes in surface proteins resulting from the preincubation of platelets with chymotrypsin, the platelets were surface radiolabeled with 1251using the iodogen method (34). The results of the surface labeling of intact and chymotrypsintreated platelets were identical to those reported in our previous studies (13) using these concentrations of chymotrypsin. The main change was in the appearance of a 66-K dalton derivative of glycoprotein IIIa on the surface of chymotrypsin-treated platelets (13). As shown recently, this 66-K dalton protein could be immunoprecipitated from detergent-solubilized, chymotrypsin-treated platelets by human anti-PIA1 antibody (11). The fibrinogen-induced aggregation of chymotrypsintreated platelets was not inhibited by prostaglandin El, forskolin, or prostacyclin. These agents, however, inhibited the ADP-induced, fibrinogen-dependent aggregation of intact (untreated) platelets (Fig. 2). We tested whether the lack of inhibition of fibrinogen-induced aggregation of chymotrypsin-treated platelets by these agents could be due to the absence of prostaglandin receptors on the platelet surface or due to degradation of the adenylate cyclase complex by chymotrypsin treatment. To test these possibilities, we measured CAMP accumulation in both intact and chymotrypsin-treated platelets. In response to prostaglandin El, forskolin, or Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 CoZZection of blood. Blood was obtained from healthy individuals with the approval of the Institutional Human Experimentation Committee at the University of Vermont, Burlington, VT. Reagents. ADP, epinephrine, prostaglandin El, forskolin, prostacyclin, albumin, chymotrypsin (grade IS), apyrase, and heparin were obtained from Sigma Chemical, St. Louis, MO. Kabi fibrinogen was purchased from Helena Laboratories, Beaumont, TX. Human washed platelets. Platelets from blood freshly collected in the anticoagulant acid-citrate dextrose were washed in the presence of apyrase and heparin by the method of Mustard et al. (22). Platelets were suspended in Tyrode solution, pH 7.35, containing 0.35% albumin, 2 mM CaC12, 1 mM MgC12, 0.36 mM NaH2P04, 135 mM NaCl, 2.68 mM KCl, 11.9 mM NaHC03, and 5.55 mM glucose. Platelet count. Platelets were counted by using a hemacytometer and an Olympus phase-contrast microscope. Treatment of platelets with chymotrypsin. Treatment of washed human platelets by chymotrypsin was carried out as previously described (13). Suspensions of washed platelets (1 x 10’ platelets/ml) were incubated with chymotrypsin for 1 h at 37OC. Untreated (intact) platelets were incubated for 1 h at 37°C without the addition of chymotrypsin. The platelets were then washed three times by centrifugation, and the platelet pellets were resuspended in Tyrode’s albumin buffer. Surface labeling of platelet proteins. Surface labeling of platelet proteins of intact and chymotrypsin-treated platelets with 12Y and sodium dodecyl sulfate polyacrylamide gel electrophoresis was carried out as previously described (11, 13, 34). CAMP accumulation. Washed, intact (untreated) platelets and chymotrypsin-treated platelets suspended in Tyrode buffer, pH 7.4, were incubated with C3H]adenine (10 &i/log platelets) for 1 h at 37OC. The platelets were then washed and resuspended in Tyrode buffer. The enzyme reaction (conversion of [3H]ATP to [3H]cAMP) was initiated by the addition of 10 PM prostaglandin E1, 1 PM prostacyclin, or 50 PM forskolin and terminated after 2 min at 37°C by the addition of 0.5 ml of 0.75 n&I CAMP and heating to 100°C for 10 min. The reaction volume was 100 ~1, and the final platelet concentration was 108/ml. ADP (50 PM) and epinephrine (10 PM) were also added at the time of initiation. The C3H]cAMP formed was isolated using a modification of the method of Salomon et al. (30) as described by Lenox et al. (14). Reaction products were centrifuged at 2,500 g for 20 min, the supernatants were applied on cation exchange columns (Bio-Rad AG50W-X8), and the [3H] precursor nucleotides were eluted with water. After the contents were eluted into alumina columns, the [3H]cAMP was eluted with 0.1 M imidazole, pH 7.4. Aliquots of the alumina eluates and [3H] -nucleotide precursor fractions were counted with 5 ml scintillation cocktail. Morphological studies. Platelets were washed twice in the presence of heparin and apyrase as described above. BY ACTIVATION OF PLATELETS BY SURFACE INTACT PROTEOLYSIS PLATELETS A 1 OOONg PQE, ADP CHYMOTRYPSIN ADP TREATED PGI, ADP f-i PLATELETS z of chymotrypsin on sponFIG. 1. Effect of various concentrations taneous aggregation of platelets by fibrinogen. Intact platelets (10’ platelets/ml) were divided into 5 equal aliquots and treated for 1 h with the concentrations of chymotrypsin shown above at 37°C. After the incubation period, platelets were washed in presence of phenylmethanesulfonyl fluoride and soybean trypsin inhibitor (23) and resuspended in Tyrode buffer containing albumin, pH 7.35 (450 ~1). Aliquots (2 x 108/ml) of platelet suspensions were tested for their ability to be aggregated by fibrinogen. Preincubation of platelets with chymotrypsin (in pg. 10’ platelets-’ *ml-l): A, 1,000; B, 750; C, 500; D, 300; and E, no chymotrypsin added. Twenty-five microliters of fibrinogen (200 pg/ml, final concn) was added to initiate platelet aggregation. prostacyclin, we found that chymotrypsin-treated platelets increased CAMP to a similar extent as did intact platelets (Table l), and no significant differences were observed between preparations (P > 0.2). The uptake of [3H] adenine by intact or chymotrypsin-treated platelets was approximately 90%, and we observed no significant differences in [3H]adenine uptake between intact and chymotrypsin-treated platelets. We also studied the ability of ADP or epinephrine to inhibit the stimulation of CAMP generation in intact and chvmotrypsin-treated platelets. Table 1 shows that the response of the adenylate cyclase system of chymotrypsin-treated platelets to epinephrine and ADP was similar to the response found with intact platelets. The treatment of platelets with 500 or 1,000 pg of chymotrypsin had no significant effect on the ability of ADP or epinephrine to inhibit stimulation of platelet adenylate cyclase by prostaglandin E1, prostacyclin, or forskolin. Since ADP acts through specific receptors to induce shape change, we have investigated whether platelet FIG. 2. Comparison of effects of stimulators of adenylate cyclase on aggregation of intact and chymotrypsin-treated platelets. Upper panel: aliquots (450 ~1) of intact platelets (3 x lO’/ml) were tested for their aggregation at 37°C by ADP and fibrinogen (200 pg/ml) under the following conditions: A, no inhibitors added; B, prostaglandin E1 (PGE1), 10 PM; C, forskolin (FSK), 50 PM; and D, prostacyclin (PGI&, 1 PM. ADP (50 PM) was added to initiate platelet aggregation. Bottom panel: aliquots (450 ~1) of chymotrypsin-treated platelets (1,000 pg 10’ platelets-‘-ml-l) at a concentration of 3 x 108/ml were aggregated by adding fibrinogen under the following conditions: A, no agents added; B, PGE1, 10 PM; C, FSK, 50 PM; and D, PG12, 1 PM. Fibrinogen (200 pg/ml) was added to initiate platelet aggregation. l ADP receptors are sensitive to proteolysis. We found that ADP was able to induce a similar shape change in both intact and chymotrypsin-treated platelets as shown by the scanning-electron micrographs in Fig. 3, A-D. These observations could be quantitated by measuring the decrease in light transmission that occurs when platelets change shape. Table 2 shows that neither the extent nor the initial velocity of ADP-induced shape change was altered by pretreatment of platelets with 500 or 1,000 pg of chymotrypsin, when compared with intact (untreated) platelets. DISCUSSION This study demonstrates that ADP is able to induce a shape change in chymotrypsin-treated platelets, a phenomenon previously reported only with intact platelets. This shape change is similar in its extent and initial velocity to that seen with intact platelets. The shape change could be blocked by stimulators of the platelet adenylate cyclase such as prostaglandin El. Therefore, the ADP receptor responsible for ADP-induced shape change appears to be present on the surface of chymotrypsin-treated platelets. However, in contrast to intact (untreated) platelets, which require ADP for platelet Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 uI8 FSK -+-I- +I--- D C B Chymotrypsin ACTIVATION OF PLATELETS BY SURFACE H553 PROTEOLYSIS TABLE 1. Inhibition by epinephrine and ADP of CAMP generation in intact and chymotrypsin-treated platelets Platelet Treatment PGE, 0.57t0.11 of [‘H]ATP % Inhibition to [3H]Cyclic Forskolin AMP:Inducing %Inhibition Agent PG12 %Inhibition (7) (3) (7) 72 2.36t0.73 (6) 0.49t0.04 (3) 60 7.15tl.43 1.94kO.60 2.37t0.93 66 0.26t0.02 (6) 80 89 (6) (3) (6) 53 57 5.71tl.49 2.8OkO.70 1.59t0.38 (5) (3) (5) 50 72 2.4OkO.80 0.51-3-0.11 0.23t0.07 (3) (3) (3) 78 90 (3) (3) (3) 42 53 6.7lt2.10 3.20t0.39 2.3620.82 (3) (3) (3) 52 64 2.11t0.40 O.BOt0.16 0.2lt0.01 (3) (3) (3) 62 90 (6) 0.30t0.03 (3) 48 0.23rtO.03 (6) 0.55t0.08 0.2620.03 0.24t0.02 0.47t0.04 0.27t0.04 0.22kO.04 Values represent means t SEM. Intact platelets and chymotrypsin-treated platelets (CTP, 500 or 1,000 gg.10’ platelets-loml-‘) were suspended in Tyrode buffer. Platelet suspensions (1 x log/ml) were first incubated with [3H]adenine for 1 h and then washed. Conversion of [3H]precursor to [3H]cyclic AMP (CAMP) by the addition of prostaglandin E1 (PGE1, 10 PM), forskolin (50 PM), or prostacyclin Iz (PG12, 1 pM) was measured in presence of 10 PM epinephrine or 50 PM ADP. Each experiment was performed in duplicate, and number of experiments is indicated in parentheses. %Inhibition of conversion of [3H]ATP to [3H]cAMP by ADP or epinephrine is also indicated. Basal 1eveIs of [3H] CAMP accumulation for intact and CTP (500 and 1,000 pg) were 0.06 * 0.01 (7), 0.09 t 0.01 (6), and 0.07 t 0.005 (3), respectively. Counts in fraction 1 of the Dowex column, representing predominantly (>80%) labeled ATP, were found to be about the same in intact and CTP (500 and 1,000 pg) (0.94 t 0.19, 1.16 t, 0.20, and 0.92 t 0.15 X lo6 cpm/108 platelets, respectively). aggregation, prior stimulation of the ADP receptor of chymotrypsin-treated platelets is not required for fibrinogen binding and aggregation. This ADP receptor appears to be coupled to the adenylate cyclase of chymotrypsin-treated platelets as shown by the ability of ADP to inhibit the stimulation of adenylate cyclase by forskolin, prostaglandin El, or prostacyclin. Whether the ADPinduced shape change and the inhibition of adenylate cyclase by ADP are the result of the stimulation of one or more types of ADP receptor(s) on chymotrypsintreated platelets presently is not known. Our findings indicate that chymotrypsin-treated platelets possess adenylate cyclase activity similar to that of intact platelets. In chymotrypsin-treated platelets, stimulators such as forskolin, which acts directly on the adenylate cyclase complex (32), or prostaglandin El and prostacyclin, were able to raise the levels of CAMP to an extent similar to that seen with intact platelets. We conclude therefore that membrane receptors for prostaglandin or prostacyclin that are coupled to the adenylate cyclase are functional in chymotrypsin-treated platelets and similar in their responsivity to those found, in intact platelets. These conclusions are based on our findings that similar levels of [3H]ATP were found in intact and chymotrypsin-treated platelets (see legend to Table 1) and that the amount of [3H]adenine taken up by the three platelet preparations was also the same. Since these are all relative measurements, however, we cannot exclude the possibility that small differences in adenylate cyclase activity do exist between intact platelets and chymotrypsin-treated platelets. The effect of chymotrypsin on isolated platelet membranes has been previously studied and shown to result in the activation of basal adenylate cyclase activity and loss of the inhibitory influence of epinephrine and GTP (4, 33). These results are clearly different from those that we have observed after chymotrypsin treatment of the surface of intact pl .atelets. We h ave found th .at both AD P and epinephrine were able to inhibit the prostaglandin El, for&Olin, or prostacyclin-stimulated adenylate cyclase activity of chymotrypsin-treated platelets. These differences are not surprising, since the guanine nucleotide regulatory proteins required for inhibition of adenylate cyclase are found on the inner surface of the plasma membrane and hence n .ot exposed to proteolysis under the conditions used in the present study. We also found that epinephrine receptors on the surface of platelets coupled to adenylate cyclase are resistant to treatment with extracellular chymotrypsin. Our findings provide direct evidence that once fibrinogen binding sites become permanently exposed on the platelet surface by iimited surface proteolysis, potent stimulators of platelet adenylate cyclase such as prostacyclin, forskolin, or prostaglandin El are unable to inhibit fibrinogen-induced aggregation. ADP-induced shape change of chymotrypsin-treated platelets, on the other hand, can be inhibited by these: agents. Our results suggest therefore that inhibition of the ADP-induced aggregation of intact platelets by increased levels of CAMP must involve interaction with events that precede the exposure of fibrinogen binding sites on the platelet surface. We provide direct evidence that CAMP does not interact with the fibrinogen binding sites themselves, as evidenced by the lack of effect of adenylate cyclase activators on the aggregation of chymotrypsin-treated platelets. Previous studies have shown that chymotrypsin-treated platelets can aggregate spontaneously in the presence of fibrinogen (6, 13, 23). The present study confirms these findings and shows that the degree of fibrinogen-induced aggregation is dependent on the concentration of chymotrypsin used during the preincubation period. Pretreatment of platelets with chymotrypsin appears to be a useful tool that allows for the dissociation of Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 Intact No agonist added + ADP + Epinephrine CTP, 500 pg No agonist added + ADP + Epinephrine CTP, 1,000 pg No agonist added + ADP + Epinephrine % Conversion Hi554 ACTIVATION OF PLATELETS BY SURFACE PROTEOLYSIS Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 FIG. 3. Scanning-electron micrographs showing ADP-induced shape change of intact and chymotrypsin-treated D: chymotrypsin-treated platelets. A: intact platelets. B: intact platelets plus ADP. C: chymotrypsin-treated platelets. platelets plus ADP. Platelets were washed and treated with chymotrypsin as described in METHODS. ADP (50 PM) was added to initiate shape change in the presence of 5 mM EGTA. One minute after addition of ADP, 1.5% glutaraldehyde (final concn) in 0.2 M phosphate buffer, pH 7.4, was added, and samples were prepared for scanningelectron microscope. ACTIVATION OF PLATELETS BY SURFACE PROTEOLYSIS H555 Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 L FIG. 3 . I 3 and D. See legend on facing page. H556 ACTIVATION OF PLATELETS 2. Effect of chymotrypsin on ADP-induced shape change TABLE Shape Change Treatment Extent, LTU Initial Velocity, LTU/min platelet shape change from the aggregation process, without blocking these responses to ADP or fibrinogen, respectively. The mechanisms underlying this dissociation may involve proteolysis of proteins that mask the fibrinogen binding site in intact, resting platelets. Proteolysis of surface proteins by extracellular proteases may prove to be useful also in studies of other receptors in a variety of cells. Several lines of investigation have demonstrated a physiological role for proteolysis as a mechanism of activation of processes ranging from blood coagulation to cell receptor activation (29). Proteolysis by extracellularly applied chymotrypsin also has been shown to have profound biological effects resulting in the differentiation of murine erythroleukemia cells (31). Several studies have implicated proteolysis in various pathophysiological states (3, 24, 28). The present study demonstrates that surface proteolysis has selective effects on specific platelet functions. It can be speculated that under certain conditions, such proteolysis may occur in vivo. Further investigations should determine whether proteolysis of platelet surface proteins plays a role in hemostatic regulation under certain physiological and/ or pathological conditions. The authors thank Dr. Dan Hendley, Dee Van Riper, Daniel De Mars, and Ann Wood for excellent technical assistance and Pat Smith for the preparation of the manuscript. E. Kornecki is a recipient of a New Investigator Research Award HL-32594 from the National Heart, Lung, and Blood Institute. A preliminary report of this work was presented at the Xth International Congress on Thrombosis and Haemostasis, San Diego, CA, July 15-19, and the abstract has been published (Thromb. Haemostas. 54: 186, 1985). Received 15 May 1985; accepted in final form 1 November 1985. onstration of granulocyte proteases in plasma of patients with acute leukemia and septicemia with coagulation defects. Blood 49: 219-231,1977. 4. FERRY, N., S. ADNOT, A. BORSODI, AND J. HANOUNE. Uncoupling by M. LAOMBE, G. GUELLAEN, proteolysis of alpha adrenergic of adenylate cyclase in human plate- receptor-mediated inhibition lets. Biochem. Biophys. Res. Commun. 108: 708-714, 1982. Evidence that changes in platelet 5. GRABER, S. E., AND J. HAWIGER. cyclic AMP levels regulate the fibrinogen receptor on human platelets. J. Biol. Chem. 257: 14606-14609, 1982. 6. GREENBERG, J., J. L. ORR, M. A, PACKHAM, M. A. GUCCIONE, E. J. HARFENIST, R. L. KINLOUGH-RATHBONE, D. W. PERRY, AND J. The effect of pretreatment of human or rabbit F. MUSTARD. platelets with chymotrypsin on their responses to human fibrinogen and aggregating agents. Blood 54: 753-765,1979. R. H., P. W. RAMWELL, AND P. J. GILMER, Cellular 7. HARRIS, mechanisms of prostaglandin action. Annu. Rev. Physiol. 41: 653668,1979. 8. HASLAM, R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser. Haematol. VI: 333-350, 1973. J., S. PARKINSON, AND S. TIMMONS. Prostacyclin in9. HAWIGER, hibits mobilisation of fibrinogen-binding sites on human ADPand thrombin-treated platelets. Nature Land. 283: 195-197, 1980. Regulation 10. INSEL, P. A., D. STENGEL, N. FERRY, AND J. HANOUNE. of adenylate cyclase of human platelet membranes by forskolin. J. BioZ. Chem. 257: 7485-7490,1982. 11. KORNECKI, E., S. Y. CHUNG, J. C. HOLT, P. TUSZYNSKI, AND S. NIEWIAROWSKI. C. S. CIERNIEWSKI, G. Identification of PIA1 alloantigen domain on a 66KDa protein derived from glycoprotein IIIa of human platelets. Biochim. Biophys. Acta 818: 285-290,1985. 12. KORNECKI, E., S. NIEWIAROWSKI, T. A. MORINELLI, AND M. KLOCZEWIAK. Effects of chymotrypsin and adenosine diphosphate on the exposure of fibrinogen receptors on normal human and Glanzmann’s thrombasthenic platelets. J. BioZ. Chem. 256: 56965701,198l. 13. KORNECKI, E., G. P. TUSZYNSKI, AND S. NIEWIAROWSKI. Inhibition of fibrinogen receptor-mediated platelet aggregation by heterologous anti-human platelet membrane antibody: significance of an Mr = 66,000 protein derived from glycoprotein IIIa. J. BioZ. Chem. 258: 9349-9356,1983. 14. LENOX, R. H., J. ELLIS, D. VAN RIPER, AND Y. H. EHRLICH. Alphag-adrenergic receptor-mediated regulation of adenylate cyclase in the intact human platelet. Evidence for a receptor reserve. Mol. Pharmacol. 15. MACFARLANE, 1. BENNETT, J. S., AND G. J. VILAIRE. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J. Clin. Invest. 64: 1393-1401, 1979. 2. BENNETT, J. S., G. VILAIRE, AND J. W. BURCH. A role for prostaglandins and thromboxanes in the exposure of platelet-fibrinogen receptors. J. CZin. Invest. 68: 981-987, 1981. 3. EGBRING, R., W. SCHMIDT, G. FUCHS, AND K. HAVEMANN. Dem- 27: l-7, 1985. E., AND D. C. B. MILLS. Inhibition by ADP of prostaglandin induced accumulation of cyclic AMP in intact human platelets. J. Cyclic Nucleotide Res. 7: l-11, 1981. 16. MARGUERIE, G. A., E. F. PLOW, AND T. S. EDGINGTON. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J. BioZ. Chem. 254: 5357-5363,1979. J. L., P. CLEZARDIN, E. JAMES, L. MCGREGOR, M. 17. MCGREGOR, DECHAVANNE, AND K. J. CLEMETSON. Identification and characterization of fragments of major glycoproteins from platelet membrane after chymotrypsin-treatment. Eur. J. Biochem. 148: 97-106, D. 1985. 18. MILLS, D. C. and Medicine, B., AND D. E. MACFARLANE. In: Platelets in Biology edited by J. C. Gordon. Amsterdam: North-Holland, 1976, p. 159-201. 19. MILLS, D. C. B., AND J. B. SMITH. The influence on platelet aggregation of drugs that affect the accumulation of adenosine 3’:5’-cyclic monophosphate in platelets. Biochem. J. 121: 185-196, 1971. 20. MORINELLI, REFERENCES PROTEOLYSIS T. A., S. NIEWIAROWSKI, E. KORNECKI, W. R. FIGY. WACHTFOGEL, AND R. W. COLMAN. Platelet aggregation and exposure of fibrinogen receptors by prostaglandin endoperoxide analogues. Blood 61: 41-49, 1973. 21. MUSIAL, J., S. NIEWIAROWSKI, D. HERSHOCK, T. A. MORINELLI, R. W. COLMAN, AND L. H. EDMUNDS, JR. Loss of fibrinogen receptors from platelet surface during simulated extracorporeal circulation. J. Lab. CZin. Med. 105: 514-522, 1985. 22. MUSTARD, J. F., D. W. PERRY, N. G. ARDLIE, AND M. A. PACKHAM. Preparation of suspensions of washed platelets from humans. Br. URES, Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 Intact (control) 4.88k0.58 43.77t5.77 Intact + PGEl 0.00 0.00 500 pg Chymotrypsin 5.00t0.57 44.33t8.35 500 pg Chymotrypsin + PGEl 0.00 0.00 40.05t7.34 1,000 pg Chymotrypsin 4.845t0.70 0.00 1,000 pg Chymotrypsin + PGEl 0.00 Values are means t SE. PGE1, prostaglandin El. Aliquots (450 ~1; 2 x 108/ml) of intact, untreated platelets or platelets treated with chymotrypsin (500 or 1,000 pg. 10’ platelets-’ *ml-‘) were incubated in a Chronolog aggregometer for 1 min at 37°C under stirring conditions. Ethylenediaminetetraacetic acid (10 ~1; 5 mM, final concn) was added. ADP (25 ~1; 50 PM, final concn) was added to initiate platelet shape change. Rate and extent of platelet shape change were measured directly from a recorder in light transmission units (LTU). BY SURFACE ACTIVATION OF PLATELETS J. Haematol. 22: 193-204, 1972. 23. NIEWIAROWSKI, S., A. Z. BUDZYNSKI, T. A. MORINELLI, T. M. BRUDZYNSKI, AND G. J. STEWART. Exposure of fibrinogen receptor on human platelets by proteolytic enzymes. 9. Biol. Chem. 256: 917-925,198l. 24. ORDINAS, A., S. MARGALL, R. CASTILLO, AND A. T. NURDEN. A glycoprotein I defect in the platelets of three patients with severe cirrhosis of the liver. Thromb. Res. 13: 297-302, 1978. 25. PEERSCHKE, E. I., M. G. ZUCKER, R. A. GRANT, J. J. EGAN, AND M. M. JOHNSON. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood 55: 841-847, 1980. 26. PLOW, E. F., AND G. A. MARGUERIE. Participation of ADP in the binding of fibrinogen to thrombin-stimulated platelets. Blood 56: 553-555,198O. 314-319,1984. 29. REICH, E., D. B. R. RIFKIN, AND E. SHAW (Editors). Proteases and biological Proliferation. H557 PROTEOLYSIS control. In: Cold Spring Harbor Conferences on Cell Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: vol. 2, 1975. 30. SALOMON, Y. Adenylate cyclase 10: 35-55, 1979. 31. SCHER, W., B. M. SCHER, AND mouse erythroleukemia them. Biophys. K. 32. SEAMON, assay. Adv. Cyclic Nucleotide Res. S. WAXMAN. Proteases stimulate cell differentiation and multiplication. Bio- Res. Commun. 109: 348-354,1982. B., W. PADGET, AND J. W. DALY. Forskolin: unique diterpene activator of adenylate cyclase in membranes and intact cells. Proc. Natl. Acad. Sci. USA 78: 3363-3367, 1981. 33. STILES, G. L., AND R. J. LEFKOWITZ. Hormone-sensitive adenylate cyclase. Delineation of a trypsin-sensitive site in the pathway of receptor-mediated inhibition. J. Biol. Chem. 257: 6287-6291, 1982. G. P., L. KNIGHT, E. KORNECKI, AND S. SRIVASTAVA. 34. TUSZYNSKI, Labeling of platelet surface proteins with [12’]-iodine by the Iodogen method. Anal. Biochem. 130: 166,1983. 35. WACHTFOGEL, Y. T., U. KUCICH, P. GLUSZKO, W. R. ABRAMS, G. WEINBAUM, H. L. SWITALSKI, S. NIEWIAROWSKI, L. H. EDMUNDS, JR., AND R. W. COLMAN. Release of neutrophil elastase during simulated extracorporeal bypass (Abstract). Circulation 70: 97, 1984. Downloaded from http://ajpheart.physiology.org/ by 10.220.32.246 on August 9, 2017 PLOW, E. F., AND G. A. MARGUERIE. Induction of the fibrinogen receptor on human platelets by epinephrine and the combination of epinephrine and ADP. J. Biol. Chem. 255: 10971-10977,198O. 28. PRINZ, R., J. FAREED, A. ROCK, G. SQUILLACI, AND J. WALLENGA. Platelet activation by human pancreatic fluid. J. Surg. Res. 37: 27. BY SURFACE