* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download + ENERGY
Fuel efficiency wikipedia , lookup
100% renewable energy wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Energy subsidies wikipedia , lookup
Regenerative brake wikipedia , lookup
Energy storage wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Zero-energy building wikipedia , lookup
International Energy Agency wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Internal energy wikipedia , lookup
Indoor air pollution in developing nations wikipedia , lookup
Low-carbon economy wikipedia , lookup
World energy consumption wikipedia , lookup
Energy harvesting wikipedia , lookup
Gibbs free energy wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Alternative energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Negawatt power wikipedia , lookup
Energy policy of Australia wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Distributed generation wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
United States energy law wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
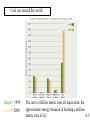
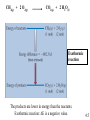
Chapter 4: Energy from Combustion What do you think of when you hear the word ENERGY? Like the energy of a crowd, you can’t see it, can’t measure it, but you know it is there. Complete Combustion – produces more energy: fuel + oxygen → CO2 + H2O + energy Incomplete Combustion – not as efficient: fuel + oxygen → CO + H2O + energy Some energy is still stored in CO, so: CO + oxygen → CO2 + H2O + energy Terms that need to be defined: Energy is the capacity to do work. Work is movement against a force (w = f x d). Heat is energy that flows from a hotter to a colder object. Temperature determines the direction of heat flow. Heat is a consequence of motion at the molecular level; temperature is a measure of the average speed of that motion. 4.1 There are many different forms of Energy: Potential – stored energy Gravitational, electrical, coiled springs Chemical – energy stored in chemical bonds and vibration/rotation of atoms Nuclear – energy stored as matter or in binding subatomic particles together Electrical – energy in motion and electrical potential of electrons Kinetic – energy of motion: Anything with mass that is moving, vibrating, or spinning Mechanical energy – moving machinery Thermal energy – motion of atoms Temperature measures average kinetic energy of matter: at absolute zero there is no motion What are units of heat? The joule (J): 1 J is the amount of energy required to raise a 1-kg object 10 cm against the force of gravity. The calorie (cal): 1 cal is the amount of heat required to raise the temperature of 1 g of water by 1 oC. 1 calorie = 4.184 J 1 kcal = 1000 cal = 1 Cal (1 dietary cal) So that 450 Cal doughnut is really 450,000 calories! 4.2 Conservation of energy (First Law of Thermodynamics) Energy may be converted from one form to another, but the total amount of energy remains constant. Energy is often measured in: Joules (J) calories (cal): 1 cal = 4.18 J Calories (Cal) = food calories 1Cal = 1000 cal Coal is used to create electricity in a power plant: 1. Combustion 2. Boil water in a closed, high pressure system 3. Turn turbine to create electricity 4.1 Coal Power Plants Convert energy to various forms: Potential (chemical energy in coal) Thermal (burning coal, heating water) Kinetic (moving steam through pipes) Mechanical (steam turns turbines) Electrical (turbines produce electricity) ??? : homes and businesses turn electrical energy back into many other forms (light, heat, mechanical...) Power plants are inevitably inefficient. Potential energy (chemical bonds) burner The First Law of Thermodynamics Energy is neither created nor destroyed, but may be transformed from one form to another. Kinetic energy gas turbine Taking random, thermal energy and transforming it into ordered work goes against the Second Law of Thermodynamics. Mechanical energy generator Electrical energy The Second Law of Thermodynamics The entropy of the universe is increasing. There is no free lunch! 4.1 The stored chemical energy in fossil fuels (coal, oil, gas,...) and wood comes from photosynthesis. Photosynthesis is basically the reverse of a combustion reaction: 6CO2 + 6H2O + energy → C6H12O6 + 6O2 versus combustion: C6H12O6 + 6O2 → 6CO2 + 6H2O + energy CO2 and H2O have very little potential chemical energy. The suns energy is stored in the products: saccharides (sugars) and oxygen. Sugar molecules link together to form other sugars, starches and cellulose. With time, pressure, and heat, dead plant material is converted into fossil fuels. Coal • Coal is a complex mixture of substances. • Although not a single compound, coal can be approximated by the chemical formula C135H96O9NS. 4.3 History of U.S. Energy Consumption by Source 1800–2008 1 EJ = 1018 J 4.3 Coal use around the world: Green = 1999 Gold = 2009 The unit is million metric tons oil equivalent, the approximate energy released in burning a million metric tons of oil. 4.3 Efficiency of Energy Conversion We are not able to completely convert energy from one form to another During the conversion, energy is often also changed into other forms Most if this energy is 'lost', since it is not in a useful form. Some examples of this are friction, heat, light, and kinetic energy lost to the environment For coal power plants: electrical energy produced Efficiency= ∗ 100% heat from fuel Each energy conversion step may be 5-90% efficient, depending on the technology used. Overall, most coal power plants are 35-50% efficient, with most energy lost as heat. Higher temperature steam more efficient For coal power plants: electrical energy produced Efficiency= ∗ 100% heat from fuel Each energy conversion step may be 5-90% efficient, depending on the technology used. Overall, most coal power plants are 35-50% efficient, with most energy lost as heat. Higher temperature steam more efficient Energy Changes at the Molecular Level The energy changes are due to the rearrangement of the atoms of the reactants and products; it is the breaking and forming of bonds that dictates if a reaction will be endothermic or exothermic. Bond energy is the amount of energy that must be absorbed to break a chemical bond. energy Breaking bonds ALWAYS requires energy! 4.6 4.6 Consider: 2 H2 + O2 2 H2O Bonds breaking 2 H–H + O=O Bonds forming 4 O–H (2 H–O–H) 4.6 Usually, reactions occur to lower the stored chemical (potential) energy. For these reactions, the potential energy lost is usually released as heat and light. Some reactions actually absorb thermal energy from the surroundings. For this to happen, the overall Entropy must increase. Entropy – measure of disorder or chaos: more chaos → more entropy. Second Law of Thermodynamics – the entropy of the universe is increasing. The entropy and energy change both determine whether or not a reaction occurs. Examples of increasing entropy (increasing disorder): solid → liquid liquid → gas cold object → warm object one compound → 2 compounds Coal – a major driving force behind the industrial revolution. Much more energy per gram than wood More abundant – not enough wood to meet energy needs There are different grades of coal Some produce more energy per gram Some are more pure carbon, while most contain many impurities, such as sulfur. Not all coal is created equal: 4.3 Some problems with coal fuel: Mine safety mine collapse dangerous gases black lung disease from breathing coal dust Environmental damage strip mining Pollution from combustion: particulates SO – lung irritant, leads to acid rain x CO production – in recent years coal has 2 accounted for up to 40% of anthropogenic CO2 production Some ways to reduce pollution: Coal washing – remove sulfur from coal before burning Gasification – process used to turn coal into CO and H2 gases, which is a much cleaner fuel, though some potential energy is lost Wet scrubbing – scrubs the exhaust gases to remove SOx. This waste can be used for commercial products, such as gypsum drywall. Hydrocarbons (HCs) – any compound containing only hydrogen and carbon Crude oil – a mixture of HCs, such as methane, ethane, propane, octane, kerosene, waxes... This mixture is separated by distillation: Smaller molecules turn into gases at lower temperatures, so each compound is boiled off at a different temperature. An Oil Refinery A Port Arthur, TX Oil Refinery 4.4 Distillation Tower: One of the drawbacks to petroleum is that it must be refined before use. 4.4 How do we use each barrel (42 gal) of petroleum? This 7.3 gal includes nonrenewable feedstocks for all plastics, pharmaceuticals, fabrics and other carbon-based products. Over 87% of each barrel is used for transportation and heating. 4.4 Heat of Combustion – energy released per mole or per gram of fuel. Different HCs have different Heats of combustion, so some produce more energy per gram than others. This energy comes from making/breaking chemical bonds. The products have lower potential energy, and the lost energy is released as heat/light Bomb calorimeters can be used to determine the heat of combustion. If you test a reaction that releases heat, the temperature of the water will increase. 4.5 Hydrocarbon fuels like methane (CH4) burn in the presence of oxygen to produce carbon dioxide and water. Energy is released in this process called combustion. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) + ENERGY When energy is released during the course of a chemical reaction, it is said to be an EXOTHERMIC reaction. The combustion of methane gas releases 50.1 kJ/g of CH4. This is the equivalent of 802.3 kJ/mol CH4. 4.5 CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) Exothermic reaction The products are lower in energy than the reactants. Exothermic reaction: E is a negative value. 4.5 Are all fuels created equal? 4.5 Gasoline Compared to combustion of octane, C H 8 18 (Octane rating) Not a pure HC, but a mixture of HCs, isomers, and additives Isomers – same formula, different structure Crude oil does not have enough octane to be used for fuel, and with mixtures, the combustive properties can be adjusted Gasoline Additives Additives improve properties combustion rate (knocking) combustion efficiency residue left in engine pollution Gasoline Additives Tetra ethyl lead (TEL) Used to be a common additive Potential problem for lead poisoning can cause sterility, insanity, loss of teeth... children more susceptible than adults Absorbed by inhalation of exhaust fumes Data shows a significant drop in lead concentrations in children after TEL was banned Gasoline Additives MTBE used to replace TEL methyl tertiarybutyl ether (H, C, and O) improves octane rating provides oxygen for combustion possible carcinogen Ethanol often used to replace MTBE Also contains only H, C and O Renewable energy source But attracts water – can cause rusting if left for prolonged periods Gasoline Additives Elimination of octane enhancing tetraethyl lead (TEL) created a need to find substitutes. MTBE, methyl tertiary-butyl ether O H3C CH3 C H H H H CH3 CH3 Ethanol (ethyl alcohol) C C H O H Human health effects of exposure to MTBE are not known. 4.7 Fuel Alternatives Biodiesel fuel use is on the rise. Made from natural, renewable sources (vegetable oils, animal fats), it can be used as pure fuel or blended with petroleum. Ethanol is renewable, but more expensive than gasoline. • Some believe it takes more energy to produce a gallon of ethanol than you will obtain from burning it. 4.9 Additives may be used to raise/lower the activation energy of the combustion. Activation energy – an energy barrier that needs to be overcome for the reaction to proceed. The activation energy controls how easily the fuel ignites, and how quickly it burns. Catalysts – something that lowers the activation energy (speeds up the reaction), but is not consumed. The catalytic converter in automobiles is a hot metal (Pt) surface that: catalyzes reactions in the exhaust gases CO + O → CO 2 2 NO → N + O x 2 2 Energy released in this converter is 'lost' as heat Historic and projected energy consumption worldwide: Note: All three projections slope upward. 4.11