* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Angiotensin-Receptor Blockade in Acute Myocardial Infarction — A
Clinical trial wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
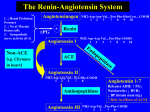
The new england journal of medicine editorials Angiotensin-Receptor Blockade in Acute Myocardial Infarction — A Matter of Dose Douglas L. Mann, M.D., and Anita Deswal, M.D., M.P.H. Beta-blockers, angiotensin-converting–enzyme (ACE) inhibitors, and aldosterone antagonists have been shown to reduce the overall risk of death as well as the risk of major nonfatal cardiovascular events when they are administered to patients with acute myocardial infarction who also have left ventricular systolic dysfunction, clinical evidence of heart failure, or both.1,2 However, there remains a sizable subgroup of patients in whom clinical heart failure worsens despite optimal medical therapy after acute myocardial infarction. Relevant to this discussion is the observation that ACE inhibitors block only 13 percent of the total production of angiotensin II in the human heart3 because of the existence of ACE-independent pathways (e.g, chymase, cathepsin, and kallikrein) that convert angiotensin I to angiotensin II. These observations provided the impetus for the development of angiotensin-receptor antagonists that offer more complete protection against angiotensin II by directly blocking the angiotensin type I receptor. However, when this therapeutic approach was tried in the Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL), in which the angiotensin-receptor antagonist losartan (at a dose of 50 mg per day) was compared with the ACE inhibitor captopril (at a dose of 150 mg per day) in high-risk patients with acute myocardial infarction,4 there was a strong trend in favor of captopril with respect to the primary end point of death from any cause (P=0.07) and a significant difference in favor of captopril with respect to the prespecified end point of death from cardiovascular causes (P=0.03). Thus, the OPTIMAAL trial raised important questions regarding the role of selective angiotensin-receptor antagonism after acute myocardial infarction. n engl j med 349;20 The Valsartan in Acute Myocardial Infarction (VALIANT) trial reported by Pfeffer et al. in this issue of the Journal 5 compared the effects of the angiotensin-receptor blocker valsartan, the ACE inhibitor captopril, and the combination of valsartan and captopril in a population of high-risk patients with clinical or radiologic evidence of heart failure, evidence of left ventricular systolic dysfunction, or both after acute myocardial infarction. A total of 14,808 patients underwent randomization in a 1:1:1 ratio to receive valsartan (titrated to 160 mg twice daily), captopril (titrated to 50 mg three times daily), or the combination of valsartan (titrated to 80 mg twice daily) and captopril (titrated to 50 mg three times daily) beginning 12 hours to 10 days after a myocardial infarction. The primary end point of the study was death from any cause, and a prespecified analysis was designed to demonstrate the noninferiority, or equivalence, of valsartan to captopril in the event that valsartan was not clearly shown to be superior in the primary analysis. During a median follow-up of 24.7 months, mortality was 19.9 percent in the valsartan group, 19.5 percent in the captopril group, and 19.3 percent in the valsartan-and-captopril group. The hazard ratio for death in the valsartan group as compared with the captopril group was 1.00 (97.5 percent confidence interval, 0.90 to 1.11; P=0.98), and the hazard ratio for death in the valsartan-andcaptopril group as compared with the captopril group was 0.98 (97.5 percent confidence interval, 0.89 to 1.09; P=0.73). The comparison of valsartan with captopril showed that these two agents were equivalent in terms of overall mortality and in terms of the rate of the composite end point of fatal and nonfatal cardiovascular events. Adverse events were less common with mono- www.nejm.org november 13, 2003 Downloaded from www.nejm.org on November 10, 2003. Copyright © 2003 Massachusetts Medical Society. All rights reserved. 1963 The new england journal therapy than with combination therapy, with hypotension and renal dysfunction being more common in the valsartan group and cough, rash, and taste disturbance being more common in the captopril group. Taken together, the results of this welldesigned and carefully performed study show that 320 mg of valsartan per day is as effective as a dose of captopril that has been shown to be superior to placebo in reducing morbidity and mortality among high-risk patients with acute myocardial infarction.1 The study by Pfeffer et al. also addressed the important question of whether more complete blockade of the renin–angiotensin system with the use of valsartan and captopril is more effective than the use of captopril alone. In contrast to two recently reported trials involving patients with heart failure, in which combination therapy with ACE inhibitors and angiotensin-receptor blockers was shown to be beneficial in terms of cardiovascular morbidity6 and mortality,7 the study by Pfeffer et al. showed that combination therapy resulted in an increase in the rate of adverse events without improving overall survival. This trial serves as an important (and painful) reminder that, in the treatment of high-risk patients with cardiovascular disease, choosing the appropriate dose of a therapeutic agent is perhaps just as important as choosing the right agent. That is, when 50 mg of losartan per day was compared with 150 mg of captopril per day in the OPTIMAAL trial, captopril was shown to be superior, and when similar doses of captopril and losartan were compared in patients with moderate-to-severe heart failure in the Evaluation of Losartan in the Elderly II trial, the results similarly favored captopril.8 In contrast, in two recently reported clinical trials in which the investigators were allowed to increase the dose of losartan gradually to 100 mg per day, there was a significant reduction in the incidence of heart failure among high-risk patients9,10; this finding raises the important question of whether higher doses of losartan might have been more effective in reducing the rates of cardiovascular events in the OPTIMAAL trial. The issue of proper doses is also germane to the question of whether the effects of valsartan can be interpreted as a “class effect” of angiotensinreceptor blockers. Aside from the obvious differences in pharmacologic profiles among the angiotensin-receptor blockers, the most compelling argument for not making assumptions regarding class effects is underscored by the different clinical outcomes in the OPTIMAAL and VALIANT trials, in 1964 n engl j med 349;20 of medicine which the determination of the correct dose of an angiotensin-receptor antagonist became a matter of life and death. How will the results of the study by Pfeffer et al. affect the care of high-risk patients after acute myocardial infarction? Given that valsartan was as effective as captopril in reducing the rates of death and other cardiovascular events, the ultimate question raised by this trial is whether high-risk patients should receive ACE inhibitors or angiotensin-receptor blockers after acute myocardial infarction. Pfeffer et al. conclude the report on their landmark study by stating that “the choice between these alternative treatments will depend on cumulative clinical experience, tolerability, safety, convenience, and cost.” The tolerability and safety of valsartan and captopril are both good for high-risk patients with acute myocardial infarction. Given that ACE inhibitors have been shown to reduce the risks of death and nonfatal cardiovascular events after acute myocardial infarction in 100,000 patients, whereas the clinical experience with angiotensin-receptor blockers has been more limited, and given that, in the United States, the cost of using valsartan at the doses used in the study by Pfeffer et al. is approximately four to six times as high as the cost of using generic captopril at the doses used in this study, ACE inhibitors remain the logical first-line therapy for high-risk patients after acute myocardial infarction. However, for those patients who cannot tolerate ACE inhibitors, the good news provided by the current trial is that there is now a safe and equally effective alternative strategy that will reduce the risks of death and adverse cardiovascular events among patients who have had an acute myocardial infarction. Dr. Mann reports having received lecture and consulting fees from AstraZeneca and lecture fees from Novartis. From the Medical Care Line, Houston Veterans Affairs Medical Center, and the Winters Center for Heart Failure Research, Baylor College of Medicine — both in Houston. 1. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1992;327:669-77. 2. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21. [Erratum, N Engl J Med 2003;348:2271.] 3. Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res 1990;66:883-90. 4. Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet 2002;360: 752-60. 5. Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, capto- www.nejm.org november 13, 2003 Downloaded from www.nejm.org on November 10, 2003. Copyright © 2003 Massachusetts Medical Society. All rights reserved. editorials pril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893906. 6. Cohn JN, Tognoni G. A randomized trial of the angiotensinreceptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75. 7. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-71. 8. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan com- n engl j med 349;20 pared with captopril on mortality in patients with symptomatic heart failure: randomised trial — the Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-7. 9. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-9. 10. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003. Copyright © 2003 Massachusetts Medical Society. www.nejm.org november 13, 2003 Downloaded from www.nejm.org on November 10, 2003. Copyright © 2003 Massachusetts Medical Society. All rights reserved. 1965