* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download rctic methane (CH4) hydrate exists on land beneath permafrost
Indian Ocean wikipedia , lookup
Future sea level wikipedia , lookup
Marine pollution wikipedia , lookup
Ocean acidification wikipedia , lookup
Physical oceanography wikipedia , lookup
Anoxic event wikipedia , lookup
History of research ships wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
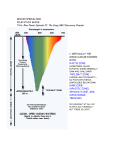
14 14 14 A rctic methane (CH4) hydrate exists on land rcticmethane methane rctic beneath permafrost ) hydrate (CH ) hydrate (CH 4 4 areas, and offshore existsonon land exists land in shelfpermafrost and beneath permafrost beneath continental margin areas,and andoffshore offshore areas, inshelf shelfand andMethane insediments. or gas hydrate, an ice-like substrate,margin consists continental margin continental mainly of light hydrocarbons (mostly sediments. Methane sediments. Methane entrapped by a substrate, rigid cage consists ofconsists orgas gashydrate, hydrate, ice-like substrate, ormethane) ananice-like water The pressure created by mainlymolecules. lighthydrocarbons hydrocarbons (mostly mainly ofoflight (mostly overlying water andbyby sediments offshore methane)entrapped entrapped rigidcage cage methane) a arigid ofof in continental margins stabilizes the CHThe watermolecules. molecules. The pressure created water pressure created bybyat 4 a temperature range well above the freezing overlying water andsediments sediments offshore overlying water and offshore exists margins asmargins frozenatice point; consequently CH continental at stabilizes theCH CH inincontinental stabilizes the 4 4 4 the seabed. The volume offreezing methane atemperature temperature rangewell wellabove abovethe the freezing a beneath range stored infrozen methane hydrates and thus the CH exists ice point;consequently consequently CH exists asasfrozen ice point; CH 4 4 4 hydrates isseabed. enormous ifvolume one considers the beneath the seabed.The The volume methane beneath the ofofmethane vast areas ofthus Arctic continental and storedshelves methane hydrates and thusthe the CH stored ininmethane hydrates and CH 4 4 margins well as permafrost areas offshore hydratesisas isenormous enormous oneconsiders considers the hydrates ififone the and on land. vastareas areas Arcticcontinental continentalshelves shelvesand and vast ofofArctic marginsasaswell wellasaspermafrost permafrostareas areasoffshore offshore margins Methane hydrate is formed in abundance andononland. land. and in deep permafrost and shallow offshore regions littleisdocumentation exists Methanebut hydrate isformed formedininabundance abundance Methane hydrate resourceand accumulations in Arctic indeep deeppermafrost permafrost andshallow shallowoffshore offshore inregarding areas and evolution through exists time and regions butits little documentation exists regions but little documentation space. Methane hydrate is only stable at low regarding resource accumulations Arctic regarding resource accumulations ininArctic temperature and high through pressure. Today’s areasand anditsitsevolution evolution throughtime timeand andsubareas seabed methanehydrate hydrateisis reservoirs remain space.Methane Methane hydrate onlystable stable low space. only atatlow elusive targets both unconventional temperature andfor high pressure. Today’ssubsubtemperature and high pressure. Today’s energy and as ahydrate natural methane emitter seabedmethane methane hydratereservoirs reservoirs remain seabed remain influencing ocean environments and elusivetargets targets forboth bothunconventional unconventional elusive for ecosystems. isnatural still contentious whether energyand andasasIta anatural methaneemitter emitter energy methane methane ascending from the ocean influencing oceanenvironments environments and influencing ocean and floor through the hydrosphere reaches ecosystems. isstill still contentious whether ecosystems. ItItis contentious whether the atmosphere. If from methane reaches methane ascending fromthe theocean ocean the methane ascending atmosphere, one can operate reaches atreaches the most floorthrough throughthe thehydrosphere hydrosphere floor fundamental level with greenhouse gas theatmosphere. atmosphere. methane reachesthe the the IfIfmethane reaches effects that one will be several times more potent atmosphere, onecan can operate the most atmosphere, operate atatthe most than CO2. level fundamental levelwith withgreenhouse greenhousegas gas fundamental effectsthat thatwill willbebeseveral severaltimes timesmore morepotent potent effects thanCO CO .. than 2 2 Top right: Sketch if climate driven change liberates theTop presently right: Opposite, above right trappedSketch methane to the driven ocean if climate Sketch if climate driven andthe atmosphere. changeliberates liberates presently change the presently trapped methane to the ocean trapped methane to the ocean Down left: andatmosphere. atmosphere. and Arctic Ocean observatories to monitor theDown stability of left: Opposite, lower left: methane hydrate fields Arctic Ocean observatories Arctic Ocean observatories offshore. monitorthe thestability stability totomonitor ofof methane hydrate fields methane hydrate fields offshore. offshore. By Jürgen Mienert, Lead of the UArctic Thematic Network on Arctic Geology, Director, Centre of Excellence for Arctic Gas Hydrate, Environment and Climate (CAGE), University of Tromsø – Norway's Arctic University 15 Methane hydrate – also called methane clathrate – would remain stable in the form of frozen methane without major changes in climate that involves a temperature increase. However, with the major projected warming trend that is underway in the Arctic regions and particularly evident in sea-ice melting during the summer, one may ask: for how long will CH4 stay trapped in methane hydrates if surface and deep ocean water masses will warm and permafrost begins to melt? These climate driven changes will liberate the presently trapped methane to the ocean and atmosphere, accelerating environmental and climate change. But how much of the Arctic methane will be released? Increasing temperatures on land and offshore will penetrate deeper and deeper into sediments at various rates depending on the flow of heat through it. Atmoshperic CH4 levels are roughly three times the preindustrial level and without any comparable scenario over the last millions of years. The methane recorded in the Greenland ice cores show distinct glacialinterglacial changes with high levels during warm periods and low levels during cold periods. As future methane concentrations may reach levels not seen for millions of years, temperatures on Earth may warm towards levels not seen in geological history for millions of years. No exact analogue for future climates exists and many research groups are trying to break the natural climate system code in order to better predict future climate change. What will the Arctic Ocean and land masses look like in the future considering polar sea ice, permafrost and methane hydrate? While the melting of sea ice observed may be the beginning of a trend of a few decades, the melting of permafrost and methane hydrates may take much longer, perhaps several hundred years. The complexity of the timing and the various response times in the ocean and on land may provide greenhouse surprises. In a world with no good climate analogue in the past, climate scientists and modelers may be confronted with many unanticipated phenomena, and surprises may be the rule and not the exception. Basic science studies of the complexity of the Arctic methane hydrate, environment and climate system are therefore needed. The Research Council of Norway selected 13 research proposals, from 139 applicants of various disciplines, which were given the status of Norwegian Centers of Excellence. The Center for Arctic Gas Hydrate, Environment and Climate (CAGE) at the University of Tromsø – Norway’s Arctic University and its partner the Norwegian Geological Survey (NGU) in Trondheim were among those selected by the Norwegian Research Council to receive 10 years of research funding. Scientists at the center will study methane release from hydrates beneath the Arctic Ocean in an effort to understand potential impacts on marine environments and global climate systems. Field programs will utilize a state-of-the-art Norwegian polar icebreaker research vessel that is planned to be launched in 2015 and will be based in Tromsø. Our aim is to, in cooperation with the Norwegian Polar Institute and others, be an important contributor to Arctic climate research internationally. Our research group is Norway’s leading in its field and, as part of Norway’s Arctic University, we have an excellent location for Arctic research. We also have a strong commitment to collaborating with relevant research entities in Russia, USA, Canada and Europe. This initiative gives the University of Tromsø the opportunity to restructure its research community and to develop unique and new collaborative scientific and technological circum-Arctic challenges. CAGE is designed to move the university from its current strong position to the forefront of international Arctic geomarine research. The main hypothesis as outlined above is that rising Arctic Ocean temperatures cause a destabilization of shallow Arctic methane hydrate reservoirs which in turn cause methane release leading to geohazards, ocean acidification and marine benthic responses at unknown rates and response times. Conceptually, CAGE aims to be a key national contributor to Arctic geomarine science. The centre creates needed science and technology sectors and enhances the ability of research in Norwegian Arctic regions to prosper by facilitating active cooperation between hydrocarbon industry, technology providers and prominent Arctic research teams.