* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neuromodulation for Failed Back Surgery
Neuropsychopharmacology wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Epidural administration wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Dydrogesterone wikipedia , lookup
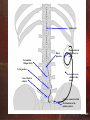
Neuromodulation for Failed Back Surgery Syndrome Part II Richard K. Osenbach, M.D. Director of Neuroscience and Neurosurgery Cape Fear Valley Health System Fayetteville, NC 8/3/2006 Spinal Opiates for Benign Pain Controversial Mixed reviews and results Reporting of outcomes non-uniform No definitive end-point for therapy 8/3/2006 Rationale of IT Drug Infusion Provide high concentration of drug at the site of interaction with spinal receptors and minimize spread to other regions in the brain 8/3/2006 History of Opiate Analgesia 1901 - intrathecal injection of morphine 1915 - antagonist of morphine discovered 1951 - 1st human use of morphine antagonists 1976 - 1st use of IT morphine in animals 1980 - spinal morphine used for cancer pain 8/3/2006 Opioid Receptors and Ligands Location of Opioid Receptors in the CNS Opioid Endogenous Dorsal horn Receptor Agonist Lamina I ß-Endorphin Substantia gelatinosa Mu (70%) Endomorphins Brainstem NucleusMet-Enkephalin caudalis Leu-Enkephalin Supraspinal Delta (20-30%) PAG Thalamic nuclei A Dynorphine StriatumDynorphine B Kappa (5-10%) Hypothalamus Limbic Nociceptin/OFQ system hORL1 Cortex Synthetic Agonists Antagonists Morphine DAMGO Naloxone ß-FNA DPDPE SNC-80 DSTBULET Naltrindole Naloxone None 8/3/2006 Mu Receptor Defined by affinity for morphine Less affinity for other receptor subtypes Most clinically important opioids selective for Mu receptor Cross react at higher doses • 1 - supraspinal 2 – spinal Most analgesic effects of systemic morphine mediated through 1 effects 70% located pre-synaptically 8/3/2006 Opioid Recptor Physiology G-protein-coupled receptor family Synthesized in DRG Second messenger using cAMP Negative coupling Inhibit cAMP via Giprotein And - opening of K+ channels - Closing of ca2+ 8/3/2006 Opioid Actions Analgesia Pruritis Urinary retention Autonomic Effects Cough suppression, orthostatic hypotension • Nucleus tractus solitarius and ambiguous, locus ceruleus Respiratory depression • Nucleus tractus solitarius, parabrachial nucleus Nausea/vomiting • Area postrema Constipation Meiosis • Superior colliculus, pretectal nuclei Endocrine effects Posterior pituitary – inhibition of vasopressin Hormonal effects – hypothalamic infundibulum Behavioral effects Amygdala, hippocampus, nucleus accumbuns, basal ganglia Motor rigidity Striatum 8/3/2006 Intraspinal Morphine Conversion Ratios 300 mg oral morphine = 100 mg parenteral morphine = 10 mg epidural morphine = 1 mg intrathecal morphine * May not be accurate at high doses 8/3/2006 Patient Selection Inclusion Criteria Opioid-responsive pain Failure of long-acting oral opioids Exclusion Criteria Spinal pathology precluding catheter placement Allergy to opiates Difficulty coming for pump refills 8/3/2006 Catheter tip Dural puncture Pump anchored with sutures or pouch Paramedian Oblique Entry V-wing anchor 5 cm of slack in catheter Loop of excess catheter under pump Catheter connector which also functions as the primary anchor 8/3/2006 Implantable Drug Pumps Programmable Constant flow 8/3/2006 Constant Flow Pump Drug delivered at constant, pre-programmed rate ADVANTAGES Unlimited life expectancy Less costly (?) DISADVANTAGES Less versatile than programmable pumps Dose changes require pump refill Flow rates influenced by physical parameters 8/3/2006 Constant Flow Pump Factors Affecting Drug Delivery Body temperature 10-13% increase in flow per 1ºC rise Geographical elevation flow increases at higher altitudes Blood pressure inversely proportional 3% change for every 10mmHg MAP Drug viscosity Q = K x (P1-P2) u Reservoir capacity flow rate calibrated for 50% capacity;4% variability at extremes of volume Pump “Dead Space” 4ml “dead volume” correction factor for concentration 8/3/2006 Programmable Pumps ADVANTAGES Maximum flexibility Variable rates Program bolus doses Alter dose by telemetry DISADVANTAGES Finite life expectancy More expensive (?) 8/3/2006 General Guidelines for IT Drug Selection Consider these issues regarding administration of intrathecal drugs: Drug stability Drug-drug compatibility for co-administration Drug-pump compatibility Effect of diluents on pump pH Choose appropriate concentration based on: Desired dose Pump capabilities Refill interval (no less than 2-4 wks) 8/3/2006 General Guidelines (cont.) DOSING STRATEGY Dose escalation with inadequate analgesia Cautious dose reduction if adequate analgesia but intolerable side effects Addition of drug; reduction of opioid dose with second analgesic EVALUATION OF THERAPEUTIC FAILURE Comprehensive patient reevaluation Assess pump and system integrity Interrogate and empty (refill assess volume) – Dye study of catheter integrity Pathophysiology of the pain 8/3/2006 Trialing for IT Therapy What do we know about screening? Multiple accepted methods No consensus as to the single best method 8/3/2006 Questions Regarding Trialing Screening method Duration of trial Drug and dose Use of placebo Systemic opioids Criteria for success 8/3/2006 Functional (Continuous) Trial ADVANTAGES Most accurately replicates permanent pump Allows for longer trials Controlled dose titration Assess starting dose for IT therapy Reduce risk of drug-related side effects Dissipates placebo effect over time Assessment of functional outcome DISADVANTGES Procedurally more complicated Requires greater expertise Higher morbidity More costly 8/3/2006 Epidural Vs. Intrathecal CRITERIA EPIDURAL INTRATHECAL Slower onset of analgesia Faster onset of analgesia Greater systemic effects Minimal systemic effects Shorter-lasting Longer-lasting Dose Higher dose to achieve effect Lower dose required (1/10 epidural dose) Adverse Effects/Risks Higher incidence of systemic side effects Risk of epidural abscess Post-LP headache Respiratory depression Meningitis Onset of Action Systemic Effects Duration of Effect 8/3/2006 Placebo Administration Rationale: reduce the likelihood of a false positive trial Normal individuals may exhibit a placebo response Difficulty interpreting placebo response A positive placebo response should not necessarily mean “no pump” Functional trialing with dose titration dissipates the placebo response over time 8/3/2006 Oral Opioids During Trial No consensus on alteration of systemic opioids during the trial Maintaining the patient on a portion of their daily dose will lessen the likelihood of withdrawal Withdrawal from systemic opioids may result in reduction in opioid-induced hyperalgesia May produce a “false positive” result 50-75% reduction in systemic dose Liberal use of “breakthrough” medication Minimal use of “breakthough” medication can be taken as one objective measure of pain relief 8/3/2006 IT Bolus (ITB) Vs. Continuous Epidural Infusion (CEI) 86 patient screened for inclusion 28 excluded from inclusion 58 patients approached 18 declined inclusion 40 patients randomized ITB (n=18) or CEI (n=19) 27 successful trial - pump implantation ITB, 67% (12/18) CEI, 79% (15/19) 3 patients lost to follow-up ITB (n=10), CEI (n=14 Anderson V, Burchiel K, Cooke B: A Prospective Randomized Trial of Intrathecal Injection vs. Epidural Infusion in the Selection of Patients for Continuous Intrathecal Opioid Therapy. Neuromodulation, 2003 8/3/2006 IT Bolus Vs. CEI No significant difference in 6 month outcomes between ITB and CEI ITB – 60% “successful” response CEI – 64% “successful” response Drug-related complications more common in ITB group (88%) vs. CEI group (70%) CEI 2.5 times more costly ($4,762 vs. 1,862) CONCLUSION: Differences in pain and functional response to long-term IT opioids among patients selected by either trial method are not large 8/3/2006 IT Bolus Vs. CEI VAS Pain Scores 100 80 60 40 20 0 Baseline 6 Months IT % Change CEI Anderson V, Burchiel K, Cooke B: A Prospective Randomized Trial of Intrathecal Injection vs. Epidural Infusion in the Selection of Patients for Continuous Intrathecal Opioid Therapy. Neuromodulation, 2003 8/3/2006 Complications Bleeding problems Spinal epidural hematoma Pump pocket hematoma/seroma Infection most often occurs at pump pocket REMOVE the system Post-dural puncture headache 20-25% incidence CSF leak 20,000 implants annually Drug-related side effects 5,000 catheter revisions annually Catheter complications Estimated revision cost $10,000 $50,000,000 yearly revision cost 8/3/2006 PA03 Update of Clinical Guidelines for the use of Intraspinal Drug Infusion in Pain Management Line 1 Morphine Hydromorphone Neuropathic Pain Morphine (or Hydromorphone) + Bupivacaine Line 2 Line 3 Morphine (or Hydromorphone) + Clonidine Morphine (or Hydromorphone) + Bupivacaine + Clonidine Fentanyl, Sufentanil, Midazolam, Baclofen Line 4 * Z i c o n o t i d e For Selected Patients Only Line 5 Line 6 Neostigmine, Adenosine, Ketorolac Ropivacaine, Meperidine, Gabapentin, Buprenorphine, Octreotide, other ** * The specific line to be determined after FDA review of NDA ** Potential spinal analgesics: Methadone, Oxymorphone, NMDA antagonists 8/3/2006 Recommended Maximum Intrathecal Dosages and Concentrations* Drug Dosage (mg/day) Concentration(mg/ml) Morphine 15 30 Hydromorphone 10 30 Bupivacaine 30 38 Clonidine 1.0 2.0 * These represent general recommendations and are dependent upon the specific patient and the clinical experience of the physician and thus, maximum dosage and/or concentrations may vary from these. 8/3/2006 Spinal Opiates Non-malignant Pain Mean morphine dose initial: 2.7 mg/day (0.3-12 mg/day) after 3.4 years: 4.7 mg/day (0.3-12 mg/day) 28 patients followed more than 4 years 64% (n=18) constant dosage history 36% (n=10) increase in morphine dose > 6mg/day after 1 year Winkellmuller et al.: J Neurosurgery 85:458-467, 1996 8/3/2006 Spinal Opiates Non-Malignant Pain U.S. experience, 1981-1992 14 authors, 156 patients 69% (107) good-excellent pain relief 75% with cancer pain – good/excellent pain relief Krames E: Spinal Administration of Opioids for Nonmalignant Pain Syndromes: A U.S. Experience 8/3/2006 Spinal Opiates Non-Malignant Pain 120 patients 63% (n=76) with FBSS or LBP Mean age: 54.0 + 11.2 years (28-79) Follow-up period mean: 3.4 + 1.3 years (0.5 - 5.7 years) Winkellmuller et al.: J Neurosurgery 85:458-467, 1996 8/3/2006 Mean Pain Scores 100 Mean VAS • 74% 80 benefit •Avg. pain reduction • 67% at 6 months •58% last follow-up 60 40 20 • 81% improved QOL 0 Before 1st FU Last FU •92% “satisfied” Winkellmuller et al.: J Neurosurgery 85:458-467, 1996 8/3/2006 Mean IT Morphine Dose (mg/day) Mean Daily Morphine Dose 5 LBP Totals 4.5 4 3.5 3 2.5 2 Initial exam First FU Last FU Winkellmuller et al.: J Neurosurgery 85:458-467, 1996 8/3/2006 Multicenter Review of Spinal Opiates Retrospective review of 429 patients 66% non-malignant pain Physician assessment global pain relief scores percent pain relief VAS scores for pain intensity ADL, overall activity level Employment Paice: J Pain Symptom Management, 1996 8/3/2006 Is it time for a nap? 8/3/2006 Global Pain Relief 43% 5% Excellent 52.4% Good 42.9% Poor 4.8% 52% Paice: J Pain Symptom Management, 1996 8/3/2006 Changes in ADL 14% 4% 82% Increased 82% No Change 14% Decreased 4% Paice: J Pain Symptom Management, 1996 8/3/2006 Daily Opiate Dosage Mean daily dose, 9.2 mg/day Initial dose higher for non-malignant pain Gradual linear dose escalation in non-malignant pain At 24 months, dosages similar in patients with nonmalignant and cancer pain Paice: J Pain Symptom Management, 1996 8/3/2006 Conclusions of Multicenter Review Nociceptive pain responds best to spinal opiates Neuropathic pain responds to spinal opiates but may require higher dosages Addition of local anesthetics may by synergistic in neuropathic pain 8/3/2006 Prospective Study - Spinal Opiates 40 patients with non-malignant pain mostly FBSS with > 3 operations Mean duration of pain, 8 + 9 years (6mos-40yrs) 30 (75%) had successful screening trial minimum of 50% pain reduction by VAS Follow-up 6, 12, 18, 24 months complete data for 20 patients followed for 2 years Outcome by VAS, CIPI, BDI, MPQ Anderson V,Burchiel K: Neurosurgery, Feb. 1999 8/3/2006 Results VAS for pain and pain coping scores remained improved CIPI and MPQ scores improved and persisted Initial morphine dose 1.96 + 1.8 mg/day, inc. to 6.0 + 7.0 at 3 months, 9.43 + 8.8 at 15 months Device complications, 20% Anderson V,Burchiel K: Neurosurgery, Feb. 1999 8/3/2006 Visual Analog Scores Mean initial VAS 80 70 78.5 ± 15.9 (39-100) 60 Decrease in VAS greatest during the initial 3 months 50 40 30 Reduction in VAS remained relatively constant 20 10 0 Initial 3-mo 6-mo 12-mo 18-mo 24-mo Anderson V,Burchiel K: Neurosurgery, Feb. 1999 8/3/2006 Medication Intake Daily IT morphine dose 25mg Mean equianalgesic opioid dose increased significantly over time initial: 1.96 ± 1.75 mg/day 24 months: 14.59 ± 20.52 mg/day Dose escalation most rapid during initial 3 months Oral narcotic intake initial: 90% (28/30) 24 months: 30% (6/30) Anderson V,Burchiel K: Neurosurgery, Feb. 1999 8/3/2006 Spinal Opiates for Benign Pain Maron J, Loeser J: The Clinical Journal Pain, 1996 Data insufficient to permit formal analysis The proper role of intraspinal opioids in the treatment of non-malignant pain cannot be determined from the existing literature Spinal opiates for benign pain should be considered experimental All patients who receive such therapy should be part of a clinical protocol 8/3/2006 Intrathecal Therapy vs. Oral Opioids, vs. Functional Restoration Program for FBSS Doleys, et. al. Interpretation as to the most effective treatment depends on the outcome measure emphasized. There is a “disconnect” between ratings of pain, disability, mood, and quality of life. The use of a multi-dimensional outcomes approach revealed a number of inconsistencies in the data which could have been overlooked using only pain ratings and patient satisfaction data. No one treatment emerged as the most effective across all of the disease specific and generic measures. Although generally “satisfied” with treatment, patients continued to report significant levels of pain, disability, and impaired quality of life 8/3/2006 Unresolved Issues How should outcome be measured? Management of tolerance Question of neurotoxicity Development of hyperalgesia Indefinite requirement for medical care 8/3/2006 The Bottom Line There can be no substitute for sound clinical judgement based on a detailed assessment of each patient ! 8/3/2006 “What do you mean, ‘It’s a bit muddy’ ?” 8/3/2006 “Men Are From Mars” 8/3/2006