* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Flaviviridae
Kawasaki disease wikipedia , lookup
Common cold wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Vaccination wikipedia , lookup
Germ theory of disease wikipedia , lookup
Orthohantavirus wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Hepatitis C wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Ebola virus disease wikipedia , lookup
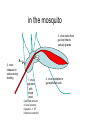
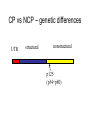
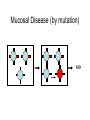
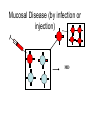
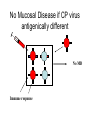
Flavi and Pestiviruses October 12, 2010 BVD, Hog cholera, Border disease Pestiviruses Flaviviridae Flaviviruses Yellow fever Japanese encephalitis St. Louis encephalitis Dengue West Nile virus (arthropods, biological vectors) Hepacviruses Hepatitis C virus West Nile Outbreaks • • • • • • • • • Israel - 1951-1954, 1957 South Africa - 1974 Romania – 1996 Italy – 1998, 2008 Russia - 1999 (human) United States –1999-2009 (equine, human) Canada - 2001-2009 (equine, human) Israel – 1998, 2000 (human) France (Rhine delta) - 2000 (equine) return from south late summer and fall spring early summer overwinter or eggs dead-end hosts amplification in birds Saskatchewan mosquito species shown to be capable of transmitting WNV • Aedes vexans (spring to fall) • Ochlerotatus flavescens, spencerii(July-August) • Culex restuans*, tarsalis* (JulyAugust) • Culiseta inornata* • Coquillettidia perturbans How does the virus overwinter and spread? • migratory birds? • overwintering mosquitos? • bird to bird transmission? – Komar et al. EID March 2003 in the mosquito 3. virus leaks from gut and infects salivary glands 4. virus released in saliva during feeding 1. virus ingested with blood meal (sufficient amount of virus must be ingested - > 105 infectious units/ml) 2. virus multiplies in gut epithelial cells in mammals virus transmitted in mosquito saliva during probing virus deposited in extra vascular tissue replication in skin and lymph nodes “flu” like symptoms amplification in extra-neural tissues PCR IgM, viremia (secretion in milk) viremia terminated by immune response crosses blood/brain barrier (repl’n in vascular endothelium, exacerbated by concurrent infections) inflammation perivascular infiltration (plasma cells and macs), cerebral edema. “neurological” signs viral damage to neurons and glia or dysfunction CSF IgM, pleocytosis, PCR equine cases of WNV neurological disease • • • • • • Ataxia 86% Depression 51% Hind limb weakness 49% Difficulty or inability to rise 46% Muscle tremors 41% Fever only 24% • Differentials: – rabies, EHV 1, EEE, WEE, botulism • 10% to 50% of horses with neurological signs die clinical signs in people • most asymptomatic • fever, “flu” like symptoms (fatigue, anorexia, nausea, vomiting, arthralgia, rash, lymphadenopathy) • encephalitis, meningoencephalitis - ataxia, painful eyes, seizures, change in mental status (confusion) case fatality rate in hospitalized patients - 10-12% risk factor for severe disease (age 50-60 yr are 10 times and >80 yr are more than 40 times likely) Petersen and Margin, WN virus: a primer for the clinician 2002. Ann Int med 137:173 unusual cases in USA • infant infected through breast milk • 2 people infected through blood transfusion • 2 laboratory workers while dissecting infected animals the Canadian experience • 2000 - 2,288 birds examined, 185 tested - no positives • 2001 - 2,807 bird carcasses from NF to Sask tested – 128 WNV infected birds from 12 health dist. in Ontario – no disease in horses or humans human cases 2003-2005 May June July Aug Sept Oct http://www.phac-aspc.gc.ca/wnv-vwn/mon-hmnsurv-archive-eng.php http://www.phac-aspc.gc.ca/wnv-vwn/mon-hmnsurv-eng.php West Nile virus in domestic birds • • • • geese ducks chickens and turkeys ostriches, emus testing for WNV - serology samples from 45 horses, Virden Manitoba (Aug-Sept, 2002) strong + cont weak + cont IgM capture ELISA PDS immunol. lab vaccines Fort Dodge Intervet – PreveNile Live Flavivirus chimera West Nile Virus – Common Client Questions • Should we vaccinate our horse – is it safe and does it work? • Can I catch WNV from a horse? • What signs might a horse show early on? • Is there a treatment? • What can we do to limit the risk to our horse? • Can our other pets get it? • When should we vaccinate our horses? Pestiviruses 1940s HCV • Systemic haemorrhagic disease - pigs (USA) BVDV • Enteric disease - calves (USA) • Congenital, neurological “hairy-shakers” (England/Wales) BDV Flaviviruses Flaviviruses Japanese encephalitis Louping ill Murray Valley Dengue West Nile St. Louis encephalitis Yellow fever Pestiviruses Hepatitis C Pestiviruses of Artydactyla bovine viral diarrhoea border disease (BVDV) (BDV) Wide host range (camelids, deer etc) hog cholera (HCV) BVDV genome and gene products UTR structural nonstructural gp53 p125 (p54+p80) Regions that show the most variation Biotypes of BVDV Based on effect on cells in tissue-culture NON-cytopathic (natural state) Cytopathic (mutant) CP vs NCP – genetic differences UTR structural nonstructural p125 (p54+p80) Biotypes - implications • Non-cytopathic – Implications for vaccines and research • Cytopathic – Mucosal disease BVDV “types” Not different serotypes!! • BVDV-1 • BVDV-2 Based on: 1. sequence differences In non-translated region of genome Does not imply differences in pathogenicity 2. Antigenic differences UTR structural nonstructural Antigenic differences between BVDV-1 and BVDV-2 Serum against VN titre against BVDV-1 BVDV-2 strains strains BVDV-1 strains 800->12,800 100->3,200 BVDV-2 strains 50->400 3,200->51,200 Pellerin et al.(1994) Virology 203:260-268 Pathogenesis - infection • Intra-species – PI carriers • • • • • Inter species Vaccine related? Artificial breeding programs Blood Persistence in acutely infected animals Pathogenesis Pathogenesis - disease in imm.competent, non-pregnant animals • Sub-clinical • Mild fever, leukopenia, decreased milk production • Mild BVD - mild erosive lesions, ulcerative stomatitis, diarrhoea, respiratory • Severe disease - lesions mimic MD, thrombocytopenia, haemorrhagic syndrome, hyphemia Pathogenesis - pregnant animals • • • • • All syndromes described above Embryonic death Abortions Birth defects Persistently infected calves Pathogenesis - PI animals • Healthy, normal • “poor-doers” • Mucosal disease Guess which one is persistently infected? Calves of the same age. From Lee et al. CVJ 38:29 consequences of having a PI animal • Loneragan et al. JAVMA, Feb 15, 2005 – PI animals more likely to be ill, require treatment or die – Incontact animals more likely to be sick, require treatment • Dieguez et al. Res Vet Sc, Aug 2009 • Correlation BVD status and respiratory disorders, mortality Mucosal disease • NCP->CP (p80) • Infection with antigenically related CP virus (mutation, herd-mate, vaccination?) • Low morbidity high mortality • High fever, depression, anorexia, diarrhoea • Ulcerative mucosal lesions • Death 2 days -> 3 weeks Mucosal Disease (by mutation) MD Mucosal Disease (by infection or injection) MD No Mucosal Disease if CP virus antigenically different No MD Immune response Diagnostic procedures • Virus isolation – Small numbers ($31.50) – Herd screening - microtitre ($10 -2->10, $6 >10) • Antigen capture ELISA – Herd screening • Serology – VN ($14) – ELISA ($5/animal) • PCR – genotyping ($62) – pooled samples ($30) Diagnostic procedures • PCR (BVDV-1 vs BVDV-2) • Immunohistochemistry or IFA BVDV-1a – Abortions ($45) – PI animals (approx. $6/animal) BVDV-1b BVDV-2 Immunohistochemistry - PI vs acute From Brad Njaa et al. 2000. J. Vet. Diag. Invest. 12:393-399. Persistently infected calf: Antigen in hair follicle epithelium Acutely infected calf: Antigen in superficial layer of epidermis (foci) Diagnostic Parameters (acute infection) infection 5-7 days incubation period antibody clinical disease virus infect. virus detectable in serum antigens in biopsy infect. virus detectable in WBC Virus det. by PCR Diagnostic parameters (abortion) • Fetus – Often no infectious virus – Submit liver, kidney, spleen, thymus for IH or IFA – Fetal antibody if late term Diagnostic parameters (PI) • Large amounts of virus in blood, serum and secretions (103-107 TCID50/ml) • Maternal antibody interferes with isolation • <3 months - submit blood • >3 months - serum • Repeat isolation in 3 months • Immunohistochemistry - follicle epithelium Herd screening Management of BVD • • • • • • Test and remove PI animals Test all new born calves for 9 months For 9-12 months segregate age groups Quarantine replacements Vaccination with MLV BVDV BVD infections may persist for some time after removal of PI animals (Collins et al. 2009) Vaccines • Inactivated or attenuated • Most (all) contain CP BVDV http://www.cattlenetwork.com/content.asp?contentid=119624 Interspecies transfer • Sheep • Wild ruminants – Natural infections (caribou: 40-100%) – Transfer (llamas, alpacas)