* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download groups - Orangefield ISD
Survey
Document related concepts
Transcript
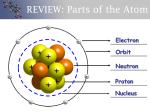
Atomic Structure and the Periodic Table Chemistry – Unit 3 Early Theories of Matter Philosophers ◦ Democritus was first to propose Atomic Theory: Matter composed of empty space through which atoms move Atoms are indivisible ◦ Aristotle rejected Atomic Theory Respected for ideas on nature, physics, astronomy, etc., so most ignored Democritus’ ideas John Dalton ◦ ◦ ◦ ◦ All matter composed of atoms Atoms can not be divided Different atoms combine to form compounds Atoms are separated, combined, or rearranged in chemical reactions ◦ Conducted convincing experiments Atom – smallest particle of an element that still retains the properties of the element ◦ Can move individual atoms around to form shapes, patterns, and simple machines Nanotechnology Subatomic Particles and the Nuclear Atom Electron discovery ◦ Sir William Crooks discovered the cathode ray Led to invention of TV Cathode ray particles carry negative charge ◦ J.J. Thomson found that mass of charged particle was much less than that of a H atom This meant Dalton was wrong and atoms are divisible Identified the electron Plum pudding model (p. 94 fig 4-9) ◦ Robert Millikan determined charge of electron Single electron carries charge of -1 Nuclear atom ◦ Ernest Rutherford concluded plum pudding model was incorrect Calculated atom consists of mostly empty space through which electrons move Concluded there is a small, dense region in the center that contains all positive charge and virtually all mass (nucleus) Nuclear model (p. 95 fig 4-12) Discovery of protons and neutrons ◦ Rutherford concluded the nucleus contains positively charged particles (protons) Protons carry a charge of +1 ◦ James Chadwick showed nucleus also contains a neutral particle in the nucleus (neutron) Mass nearly equal to proton Neutral charge ◦ Electrons are held within atom by attraction to positively charged nucleus ◦ Number of protons equals number of electrons How Atoms Differ Atomic number ◦ Defined as the number of protons in an atom ◦ Determines element’s position on periodic table ◦ Atomic number = proton # = electron # Isotopes - all atoms of an element have same number of protons and electrons, but number of neutrons differ (isotopes) Mass number – sum of proton # and neutron # ◦ Number of neutrons = mass # - atomic # Mass of individual atoms ◦ Protons and neutrons have approx. same mass ◦ Electrons are MUCH smaller ◦ B/c the masses are so small (must use scientific notation, which is cumbersome), chemists developed a standard for measurement Carbon-12 atom Exactly 12 atomic mass units (amu) 1 amu is 1/12 the mass of carbon-12 atom ◦ Atomic mass of an element is weighted average mass of the isotopes of that element Unstable Nuclei and Radioactive Decay Radioactivity ◦ Chemical reactions involve only an atom’s electrons Nucleus remains unchanged Atom identity does not change ◦ Nuclear reactions involve change in atom’s nucleus Atom of one element changes into atom of another element ◦ Radioactivity – some substances spontaneously emit radiation ◦ Radiation – rays and particles emitted by the radioactive material ◦ Radioactive atoms emit radiation b/c their nuclei are unstable ◦ Radioactive decay - unstable nuclei lose energy by emitting radiation spontaneously Types of radiation ◦ Experiment conducted by scientists in late 1800s determined some radiation was deflected toward positively charged plate, some toward negatively charged plate, some not at all ◦ Alpha radiation – radiation deflected toward negatively charged plate Alpha particles 2 protons, 2 neutrons +2 charge Equivalent to He-4 nucleus Represented by α ◦ Beta radiation – radiation deflected toward positively charged plate Fast-moving electrons called beta particles Beta particle is 1 electron with -1 charge Represented by β ◦ Gamma rays – high-energy radiation that possess no mass and no charge Represented by γ B/c massless, emission of gamma rays can not result in formation of new atom ◦ Nuclear stability Primary factor is ratio of neutrons to protons Atoms w/ too few/many neutrons are unstable Few radioactive atoms in nature ◦ Nuclear equation – shows atomic #, mass #, and particles involved Both mass # and atomic # are conserved Development of the Modern Periodic Table Modern Periodic Table ◦ Periodic law – states that there is a periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number ◦ Arranged in order of increasing atomic number into a series of columns, called groups, and rows, called periods ◦ Groups are labeled 1-8, followed by A and B “A” groups are the representative elements b/c they possess a wide range of chemical and physical properties “B” groups are the transition elements ◦ Classifying elements – 3 main classifications for elements Metals Located on left side of periodic table (H is exception) Shiny, solid at room temp., good conductors, malleable, ductile Group 1A (except H) – alkali metals Very reactive Group 2A – alkaline earth metals Reactive Transition elements Located in the middle section of the periodic table Transition metals Inner transition metals – very bottom of the table to save space Lanthanide series Actinide series Nonmetals Located in upper right side of table Generally gases or brittle, dull solids, poor conductors Only bromine is liquid at room temp. Group 7A – halogens Highly reactive Group 8A – noble gases Unreactive Metalloids Located on border of stair-step line Physical and chemical properties of both metals and nonmetals Classification of the Elements Organizing the elements by electron configuration ◦ Valence electrons – one of the most important relationships in chemistry Atoms in the same group have similar chemical properties b/c they have the same number of valence electrons ◦ Valence electrons and period – the energy level of an element’s valence electrons indicates its period Example: Li’s valence electron is in 2nd energy level and Li is in period 2; Ga’s electron configuration is [Ar]4s23d104p1, its valence electrons are in the fourth energy level, and it’s found in the 4th period ◦ Valence electrons and group number – representative element’s group number and valence electrons are related Noble gases have 8 valence electrons (except He) The s-, p-, d-, and f-block elements – b/c there are 4 different energy sublevels (s, p, d, f), the periodic table is divided into 4 distinct blocks (Fig 6-10) ◦ S-block – groups 1A, 2A, and H and He ◦ P-block – groups 3A – 8A Group 8A (noble gases) are unique b/c of their stability; they undergo virtually no chemical reactions ◦ D-block – transition metals ◦ F-block – inner transition metals ◦ Period patterns Period 1 contains only s-block Periods 2 and 3 contain s- and p-block Periods 4 and 5 contain s-, p-, and d-block Periods 6 and 7 contain s-, p-, d-, and f-block Periodic Trends Atomic radius ◦ Trends within periods – general decrease in atomic radii left-to-right No additional electrons come between the valence electrons and nucleus ◦ Trends within groups – general increase in atomic radii moving down Outermost orbital increases in size along w/ increasing principal energy level, making atom larger ◦ Fig 6-12 Ionic radius ◦ Atoms can gain or lose electrons to form ions ◦ Ion – atom or bonded group of atoms that has a positive or negative charge ◦ When atoms gain electrons and form negatively charged ions, they always become larger ◦ When atoms lose electrons and form positively charged ions, they always become smaller Ionization energy – energy required to remove an electron from a gaseous atom ◦ First ionization energy - energy required to remove the first electron from an atom ◦ Indication of how strongly an atom’s nucleus holds onto its valence electrons High ionization energy – atom has strong hold on electrons and are less likely to form positive ions ◦ Trends within periods and groups Fig 6-17 ◦ Octet rule – atoms tend to gain, lose, or share electrons in order to acquire a full set of 8 valence electrons Elements on right side of periodic table tend to gain electrons Form negative ions Elements on left side of table tend to lose electrons Form positive ions Electronegativity – indicates the relative ability of its atoms to attract electrons in a chemical bond ◦ Fig 6-18 Properties of s-Block Elements Representative Elements ◦ The lower the ionization energy, the more reactive the metal Metal groups – reactivity increases as the atomic number increases ◦ The higher the ionization energy, the more reactive the nonmetal Nonmetal groups – reactivity decreases as the atomic number increases Hydrogen ◦ Placed in group 1A only b/c it has 1 valence electron ◦ Has metallic and nonmetallic properties, so is not considered part of any group Group 1A: Alkali Metals ◦ Lose 1 valence electron and form a 1+ ion ◦ Soft ◦ Lithium Least reactive alkali metal Long-lasting batteries Drug to treat bipolar disorders ◦ Sodium and potassium Fireworks Fertilizers Group 2A: Alkaline Earth Metals ◦ ◦ ◦ ◦ Form compounds with oxygen (oxides) Shiny solids that are harder than alkali metals Lose 2 valence electrons to form 2+ ions Calcium Healthy bones and teeth Calcium carbonate Main ingredient in limestone, chalk, and marble Antacid tablets Abrasives, such as toothpaste ◦ Magnesium – alloys of Mg w/ Al and Zn are strong as steel but lighter ◦ Barium – used in paints, glass; used as diagnostic tool for internal medicine Properties of p-Block Elements Group 3A: The Boron Group ◦ Boron Borosilicate glass for cookware Borax - cleanser Boric acid – disinfectant ◦ Aluminum – most abundant metal Aluminum sulfate in anti-perspirants ◦ Gallium Thermometers Blue lasers Group 4A: The Carbon Group ◦ Carbon Organic chemistry studies C-containing compounds Inorganic chemistry studies all others Mineral – inorganic element found in crystals Ore – material from which minerals can be removed Diamond and graphite are allotropes of C Allotropes – forms of an element in the same physical state that have different structures and properties ◦ Silicon Semi-conductors Sand and glass Group 5A: The Nitrogen Group ◦ Nitrogen – 78% of earth’s atmosphere Ammonia TNT, nitroglycerine ◦ Phosphorus Matchbox striking surface Fertilizers Fertilizers containing phosphates harm environment ◦ Arsenic – toxin used in poisons ◦ Bismuth – main ingredient in Pepto Bismol Group 6A: The Oxygen Group ◦ Oxygen – most abundant element in earth’s crust Bonds with most elements ◦ Sulfur SO2 – reacts w/water vapor to form acid rain ◦ Selenium Vitamins Solar panels Photocopiers Group 7A: The Halogens ◦ Form compounds w/ almost all metals (salts) ◦ Fluorine – most electronegative element, so greatest tendency to attract electrons Toothpaste Drinking water ◦ Chlorine Disinfectant Bleach HCl in stomach used to digest food PVC ◦ Iodine Maintains healthy thyroid gland Kills bacteria Properties of d-Block and f-Block Elements Transition Metals ◦ Silver is best conductor ◦ Iron and titanium are used as structural materials b/c of their strength ◦ Chromium is hardest 6 unpaired electrons ◦ Magnetism – ability of a substance to be affected by a magnetic field Moving electron creates magnetic field; b/c paired electrons move in opposite directions, their magnetic fields tend to cancel ◦ Sources of transition metals U.S. imports more than 60 materials that are classified as “strategic and critical” Inner Transition Metals ◦ Lanthanide series – silvery metals w/ relatively high melting points ◦ Actinide series Radioactive Transuranium element – atomic number >92 Plutonium is used as fuel in nuclear power plants Americium – used in smoke detectors