* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Rotational spectroscopy
Canonical quantization wikipedia , lookup
Schrödinger equation wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Matter wave wikipedia , lookup
Spherical harmonics wikipedia , lookup
Particle in a box wikipedia , lookup
Electron configuration wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Atomic orbital wikipedia , lookup
Tight binding wikipedia , lookup
Wave–particle duality wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Rigid rotor wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Atomic theory wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
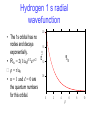
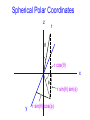
Spectroscopy Lecture 3 Hydrogen Atom Rotational Spectroscopy NC State University Experimental observation of hydrogen atom • Hydrogen atom emission is “quantized”. It occurs at discrete wavelengths (and therefore at discrete energies). • The Balmer series results from four visible lines at 410 nm, 434 nm, 496 nm and 656 nm. • The relationship between these lines was shown to follow the Rydberg relation. The Solar Spectrum • There are gaps in the solar emission called Frauenhofer lines. • The gaps arise from specific atoms in the sun that absorb radiation. Atomic spectra • Atomic spectra consist of series of narrow lines. • Empirically it has been shown that the wavenumber of the spectral lines can be fit by where R is the Rydberg constant, and n1 and n2 are integers. Note that n2 > n1. Schrödinger equation for hydrogen: The kinetic energy operator The Schrö dinger equation in three dimensions is: 2 2 h – + V = E 2 The operator del-squared is: 2 2 2 = 2+ 2+ 2 x y z 2 The procedure uses a spherical polar coordinate system. Instead of x, y and z the coordiantes are q, f and r. The Bohr radius The quantity a0 = 4pe0h2/me2 is known as the Bohr radius. The Bohr radius is a0 = 0.529 Å . Since it emerges from the calculation of the wave functions and energies of the hydrogen atom it is a fundamental unit. In so-called atomic units the unit of length is the Bohr radius. So 1 Å is approximately 2 Bohr radii. You should do a dimensional (unit) analysis and verify that a0 has units of length! Energy levels of hydrogen atom • The energy levels of the hydrogen atom are specified by the principal quantum number n: • All states with the same quantum number n have the same energy. • All states of negative energy are bound states, states of positive energy are unbound and are part of the continuum. The Rydberg Constant • The energy levels calculated using the Schrö dinger equation permit calculation of the Rydberg constant. • One major issue is units. Spectroscopists often use units of wavenumber or cm-1. At first this ~ seems odd, but hn = hc/l = hcn where n is ~the value of the transition in wavenumbers. in cm-1 The simple form • Using the Rydberg constant the energy of the hydrogen atom can be written as: ~ R E= – 2 n ~ where R = 109,636 cm-1 Shells and subshells • All of the orbitals of a given value of n for a shell. • n = 1, 2, 3, 4 .. correspond to shells K, L, M, N… • Orbitals with the same value of n and different values of l form subshells. • l = 0, 1, 2, ... correspond to subshells s, p, d … • Using the quantum numbers that emerge from solution of the Schrö dinger equation the subshells can be described as orbitals. Spherical harmonics These are the spherical harmonics , which are solutions of the angular Schrodinger equation. Hydrogen 1 s radial wavefunction • n = 1 and l = 0 are the quantum numbers for this orbital. 1.5 R1,0 • The 1s orbital has no nodes and decays exponentially. • R1s = 2(1/a0)3/2e-r/2 r = r/a0 2.0 Y1s 1.0 0.5 0 2 4 r 6 8 10 The Radial Distribution in Hydrogen 2s and 2p orbitals Rn, 0.6 Y2s Y2p 0.4 0.2 0.0 0 2 4 r 6 8 10 The Dipole Moment Expansion The permanent dipole moment of a molecule oscillates about an equilibrium value as the molecule vibrates. Thus, the dipole moment depends on the nuclear coordinate Q. Q = 0 + Q + ... Q where is the dipole operator. Rotational Transitions Rotational transitions arise from the rotation of the permanent dipole moment that can interact with an electromagnetic field in the microwave region of the spectrum. Q = 0 + Q + ... Q Spherical Polar Coordinates z r q r cos(q) x f r sin(q) sin(f) y r sin(q) cos(f) Rotation in two dimensions Our first approach is classical. The angular momentum is Jz = pr. Jz r m p Using the deBroglie relation p = h/l we also have a condition for quantization of angular motion Jz = hr/l. Classical Rotation In a circular trajectory Jz = pr and E = Jz2/2I. I is the moment of inertia. Diatomic I = r2 Mass in a circle I = mr2. r m r m1 m2 m 1m 2 Reduced mass = m + m 1 2 The 2-D rotational hamiltonian • The wavelength must be a whole number fraction of the circumference for the ends to match after each circuit. • The condition 2pr = Ml combined with the deBroglie relation leads to a quantized expression,Jz = Mh where M is a quantum number for rotation in two dimensions. • The hamiltonian is: Energy level spacing Energy levels Energy Differences of DJ = ± 1 Quantization of rotational motion: solution of the f equation The corresponding wavefunctions are: with the constraint that: Since the energy is constrained to values Jz2/2I we find that The wavefunctions of a rigid rotor are called spherical harmonics The solutions to the q and f equation (angular part) are the spherical harmonics Y(q,f )= Q(q)F(f) Separation of variables using the functions Q(q) and F(f) allows solution of the rotational wave equation. 2 h Y 1 1 sinq Y – + q 2I sin2q 2 sinq q 2 = EY We can obtain a q and f equation from the above equation. Rotational Wavefunctions J=0 J=1 J=2 These are the spherical harmonics YJM, which are solutions of the angular Schrodinger equation. The form of the spherical harmonics Including normalization the spherical harmonics are Y00 = 1 4p 0 3 cosq Y1 = 4p ±1 Y1 = Y20 = ±1 2 Y = 3 sinqe ±if 2 Y2 = 8p 5 3cos2q – 1 16p 15 sinqcosqe ±if 8p 15 sin2qe ±2if 32p The form commonly used to represent p and d orbitals are linear combinations of these functions Euler relation Linear combinations are formed using the Euler relation e ±if = cosq ±isinq if –if e – e sinq 2i if –if cosq e + e 2 Projection along the z-axis is usually taken using z = rcosq. Projection in the x,y plane is taken using x = rsinqcosf and y = rsinqsinf Solutions to the 3-D rotational hamiltonian • There are two quantum numbers J is the total angular momentum quantum number M is the z-component of the angular momentum • The spherical harmonics called YJM are functions whose probability |YJM|2 has the well known shape of the s, p and d orbitals etc. J = 0 is s , M = 0 J = 1 is p , M = -1, 0 , 1 J = 2 is d , M = -2 , -1, 0 , 1, 2 J = 3 is f , M = -3 , -2 , -1, 0 , 1, 2, 3 etc. The degeneracy of the solutions • The solutions form a set of 2J + 1 functions at each energy (the energies are • A set of levels that are equal in energy is called a degenerate set. J=3 J=2 J=1 J=0 Orthogonality of wavefunctions • The rotational wavefunctions can be represented as the product of sines and cosines. • Ignoring normalization we have: • s 1 • p cosq, sinqcosf, sinqsinf • d 1/2(3cos2q - 1), cos2qcos2f , cos2qsin2f , cosqsinqcosf , cosqsinqsinf • The differential angular element is sinqdqdf/4p over • the limits q = 0 to p and f = 0 to 2p. • The angular wavefunctions are orthogonal. Orthogonality of wavefunctions • For the theta (q) integrals we can use the substitution • x = cosq and dx = sinqdq • For example, for s and p-type rotational wave functions, for the theta integral we have • Using these substitutions we can turn all of these integrals into polynomials. The moment of inertia The kinetic energy of a rotating body is 1/2Iw2. The moment of inertia is given by: I= mr i=1 2 i i The rigid rotor approximation assumes that molecules do not distort under rotation. The types or rotor are (with moments Ia , Ib , Ic) - Spherical: Three equal moments (CH4, SF6) (Note: No dipole moment) - Symmetric: Two equal moments (NH3, CH3CN) - Linear: One moment (CO2, HCl, HCN) (Note: Dipole moment depends on asymmetry) - Asymmetric: Three unequal moments (H2O) Polyatomic Molecules • There are 3N total degrees of freedom in a molecule that contains N atoms. • There are three translational degrees of freedom. These correspond to motion of the center of mass of the molecule. • In a linear molecule there are two rotational degrees of freedom. In a non-linear molecule there are 3 rotational degrees of freedom. • The remaining degrees of freedom are vibrational. Spectroscopy of atomic hydrogen • Spectra reported in wavenumbers (cm-1) • Rydberg fit all of the series of hydrogen spectra with a single equation, n=R 1 – 1 n n • Absorption or emission of a photon of frequency n occurs in resonance with an energy change, DE = hn (Bohr frequency condition). • Solutions of Schrö dinger equation result in further selection rules. 2 1 2 2 Spectroscopic transitions • A transition requires a transfer from one state with its quantum numbers (n1, l1, m1) to another state (n2, l2, m2). • Not all transitions are possible: there are selection rules, Dl = 1, m = 0, 1 • These rules demand conservation of angular momentum. Since a photon carries an intrinsic angular momentum of 1. The interaction of electromagnetic radiation with a transition moment The electromagnetic wave has an angular momentum of 1. Therefore, an atom or molecule must have a change of 1 in its orbital angular momentum to conserve this quantity. This can be seen for hydrogen atom: Electric vector of radiation l=0 l=1 A propagating wave of electromagnetic radiation of wavelength l has an oscillating electric dipole, E (magnetic dipole not shown) l E The oscillating electric dipole, E, can induce an oscillating dipole in a molecule as the radiation passes through the sample l E The oscillating electric dipole, E, can induce an oscillating dipole in a molecule as the radiation passes through the sample l n=1 ∆E = hc/l n=0 The type of induced oscillating dipole depends on l. If l corresponds to an electronic energy gap, then radiation will be absorbed, and a molecular electronic transition will result The oscillating electric dipole, E, can induce an oscillating dipole in a molecule as the radiation passes through the sample l LUMO ∆E = hc/l HOMO If l corresponds to a electronic energy gap, then radiation will be absorbed, and an electron will be promoted to an unfilled MO The oscillating electric dipole, E, can induce an oscillating dipole in a molecule as the radiation passes through the sample l R v=1 O R ∆E = hc/l R v=0 O R The type of induced oscillating dipole depends on l. If l corresponds to a vibrational energy gap, then radiation will be absorbed, and a molecular vibrational transition will result Rotational spectroscopy Rotational Transitions • Electromagnetic radiation can interact with a molecule to change the rotational state. • Typical rotational transitions occur in the microwave region of the electromagnetic spectrum. • There is a selection rule that states that the quantum number can change only by + or - 1 for an allowed rotational transition (DJ = 1). J=2 J=1 J=0 Rotational Transitions • Treat the electromagnetic wave as having a polarization along x, y, or z. • The transition integral is not zero in this case since the zpolarized transition is matched to the pz rotational orbital. The total wave function in calculation of transition moment The total wave function can be factored into an electronic, a vibrational and a rotational wave function. = el vYJM M rot = = = *vYJM *el eld el vYJMd nuc *vYJM 0 vYJMd nuc *v vdQ YJM 0YJMsinqdqdf The rotational transition moment The transition moment is a dipolar term that connects two states. Here we are considering rotational states that have quantum numbers J, M in the initial state and J’,M’ in the final State Mrot = 0 YJMYJMsinqdqdf The electronic integral gives 0, the permanent dipole moment. The vibrational wave functions are normalized and the integral is 1. Interaction with radiation For example, if an oscillating electromagnetic field enters as E0cos(wt)cos(q) such that hw is equal to a rotational energy level difference, the interaction of the electric field with the transition moment is 2p MrotM=rotE=0 0 p cos q YJMsin q dqdf * J+1,M Y 0 0 Interaction with radiation The choice of cos(q) means that we consider z-polarized microwave light. In general we could consider x- or y-polarized as well. x sin(q)cos(f) 0 = X i + Y j + Zk y sin(q)sin(f) 0 = 0 sinqcosfi + sinqsinf j +cosqk z cos(q) Pure rotational spectra • A pure rotational spectrum is obtained by microwave absorption. • The range in wavenumbers is from 0-200 cm-1. • Rotational selection rules dictate that the change in quantum number must be DJ = ± 1 • A molecule must possess a ground state dipole moment in order to have a pure rotational spectrum. Energy level spacing Energy levels Energy Differences of DJ = ± 1 The rotational constant The spacing of rotational levels in spectra is given by DE = EJ+1- EJ according to the selection rule The line spacing is proportional to the rotational constant B In units of wavenumbers (cm-1) this is: A pure rotational spectrum ~ 2B A pure rotational spectrum is observed in the microwave range of electromagnetic spectrum. Key Points • The vibrational energy levels are given by: The energy levels have a degeneracy of 2J + 1 • The wave functions are the spherical harmonics. Transition energies are given by: • B is the rotational constant • Rotational spectra consist of a series lines separated by 2B.