* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download drugs that alter dopamine nerve structure or function
Chemical weapon wikipedia , lookup
Vesicular monoamine transporter wikipedia , lookup
Chemical synapse wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Jack DeRuiter, Principles of Drug Action 2, Fall 2001
DRUGS THAT ALTER DOPAMINE NERVE STRUCTURE OR FUNCTION
MC Objective: Characterize the chemical and pharmacologic properties of "DA releasers"
such as Amantadine and the amphetamines)
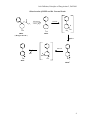
NH2
Amantadine
•
•
•
•
•
•
•
•
Amantadine is a basic amine (pKa 10.8) and predominantly ionized at physiologic
pH.
Amantadine has a lipophilic carbon "cage structure" that enhances tissue penetration
and sterically blocks metabolism (no AMO or other deamination metabolism)
Amantadine promotes DA release from presynaptic nerves (activity is dependent on
intact and functional DA nerves. Its activity is enhanced when administered with LDOPA (greater DA formation)!
Amantadine also delays the reuptake of DA in functional DA nerves
Amantadine has a rapid onset : within 2 weeks
Amantadine has lower efficacy (because of its mechanism of action) but fewer side
effects that L-DOPA
Amantadine is eliminated unchanged renally and therefore should be used with
caution in renal impairment (reduce dose!)
Amphetamines (see structures below) can release DA, as well as inhibit DA reuptake
and weakly block MAO metabolism by competitive inhibition this enzyme.
MC Objective: Characterize the chemical and pharmacologic properties of inhibitors of
DA release (GHB and its lactone)
HO
HO
O
Gamma-Hydroxybutyric Acid (GHB)
•
•
O
O
Butyrolactone
GHB and its lactone form specifically block DA release (not other NTs) by an
unknown mechanism
This drug, particularly in its lipophilic lactone form can pass biologic membranes
including the BBB
1
Jack DeRuiter, Principles of Drug Action 2, Fall 2001
MC Objective: Characterize the chemical and pharmacologic properties of the natural
product Reserpine (from Rauwolfia serpentina), a DA depletor
N
N
H H
H
OCH3
H
CH3OOC
OOC
H
OCH3
OCH3
OCH3
Reserpine
•
•
•
•
•
•
•
Reserpine depletes NE from dopaminergic (and adrenergic and serotonergic nerves)
by inhibiting the Mg-ATPase transporter responsible for sequestering DA (and NE
and 5-HT) in storage vesicles. The DA not stored is largely metabolized by MAO.
This results ultimately in depletion of intraneuronal stores of neurotransmitter.
Based on its mechanism of action, reserpine has been used for the treatment of
schizophrenia and hypertension.
Reserpine is used only infrequently now because its non-specific acxtions result in an
array of undersired peripheral and central side effects.
Reserpine in solution may undergo hydrolysis, indole decomposition (light-mediated)
and epimerization. Show the epimerization product and why it occurs.
Reserpine readily passess the BBB by diffusion and enters the CND due to it's
lipophilicity.
Why does the duration of action of reserpine exceed its plasma half-life?
Reserpine is metabolized by esterase-mediated hydrolysis and oxidative Odemethylation. Propose structures for these metabolites.
MC Objective: Characterize the chemical and pharmacologic properties of the inhibitors
of DA reuptake: Note that the structures required to block DA reuptake differ
significantly from those required to block NE or 5-HT reuptake!
O
NHR
H
S
N CH(CH )
32
CH3
CH3
R = H: Amphetamine
R = CH3 : Methamphetamine
Cl
N
CH3
N
CH3
CH3
CH2CH3
Bupropion
Tandamine
H3C
N
O
N
CH3
NH2
COOCH3
Nomifensine
O
Cocaine (and related compounds)
2
Jack DeRuiter, Principles of Drug Action 2, Fall 2001
•
•
•
Many of these drugs have multiple pharmacologic actions (amphetamines and
cocaine) in addition to inhibition of DA reuptake.
Cocaine primarily is a drug of abuse that has a short half-life due to rapid metabolic
inactivation by ester hydrolysis and OND
The actions of some the DA reuptake inhibitors (Bupropion and Nomifensine) will be
covered in more when inhibition of NT reuptake is discussed generally.
MC Objective: Characterize the chemical and pharmacologic properties of compounds
capable of destroying DA neurons.
•
•
6-Hydroxydopamine and xylamine are DA depletors in PNS (except the adrenal
medulla) and CNS
Irreversibly destroy sympathetic and DA neurons via formation of reactive
metabolites that are capable of alkylating nucleophilic residues on nerve enzymes and
proteins and rendering the nerve non-functional:
OH
O
NH2
NH2
[O]
HO
HO
OH
O
6-Hydroxydopamine
"Reactive Quinone"
Destruction of
DA nerve terminals
Cl
Chemical
N
CH3
Xylamine
•
CH3
N
CH3
CH3
Aziridnium Ion
MPTP is a by-product or contaminant in the synthesis of the narcotic MPPP
(meperidine analogue referred to as "designer heroin"
1. MPTP binds selectively to MAO-B which is highly concentration in the SN
2. Glial and 5-HT cells convert MPTP to MPDP+ which can diffuse iout an
disproportionate to MPP+
3. DA and NE terminals accumulate MPP+ through catecholamine uptake.
4. Neuromelanin of SN neurons bind MPP+
5. MPP+ rapidly accumulates in mitochondria where it inhibits NADH
dehydrogenase resulting in ATP depletion and cell death
3
Jack DeRuiter, Principles of Drug Action 2, Fall 2001
Bioactivavtion of MPPP and DA Neuronal Death
O
Elimination
O
C3H7
MAO-B
-OOCC3H7
H
N
N
N
CH3
CH3
CH3
MPPP
("Designer heroin")
OH
MPTP
H2O
MAO-B
H2O
N
H
HO
CH3
N
N
CH3
CH3
+
MPP
+
MPDP
4