* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Assessing the health of European rivers using
Survey
Document related concepts
Biogeography wikipedia , lookup
Introduced species wikipedia , lookup
Occupancy–abundance relationship wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Biological Dynamics of Forest Fragments Project wikipedia , lookup
Island restoration wikipedia , lookup
Restoration ecology wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Habitat conservation wikipedia , lookup
Transcript
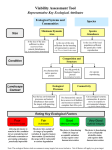
Fisheries Management and Ecology, 2007, 14, 381–392 Assessing the health of European rivers using functional ecological guilds of fish communities: standardising species classification and approaches to metric selection R. A. A. NOBLE & I. G. COWX Hull International Fisheries Institute, University of Hull, Hull, UK D. GOFFAUX & P. KESTEMONT University of Namur, Namur, Belgium Abstract The functional ecological guild approach is the cornerstone for the development of Indices of Biotic Integrity and multi-metric indices to assess the ecological status of aquatic systems. These indices combine metrics (unit-specific measures of a functional component of the fish community known to respond to degradation) into a single measure of ecological assessment. The guild approach provides an operational unit linking individual species characteristics with the community as a whole. Species are grouped into guilds based on some degree of overlap in their ecological niches, regardless of taxonomic relationships. Despite European fish species having been classified into ecological guilds, classification has not been standardised Europe-wide or within the context of classifying species into guilds from which metrics can be developed for ecological assessment purposes. This paper examines the approach used by the EU project FAME to classify European fish species into consistent ecological guilds and to identify suitable metrics as basic tools for the development of a standardised ecological assessment method for European rivers to meet the requirements of the Water Framework Directive. KEYWORDS: ecological guild, Europe, fish, Index of Biological Integrity, metrics, rivers. Introduction The Water Framework Directive as the basis for harmonised ecological assessment methods Implementation of the European Union Water Framework Directive (WFD) (EU 2000) as the standard framework for water management within the European Union requires harmonisation of ecological assessment and the interpretation of the ecological status of water bodies. Within the WFD, fish are one of the key quality elements used to describe the ecological status of rivers. Fish are good indicators of ecological status as they occupy a wide range of ecological niches and operate over a variety of spatial scales (Simon 1999). Consequently, fish have been used to develop community- based indices that integrate a number of measures (metrics) of functional community structure, linking the ecological functions and requirements of different species to the impacts of human pressures on the structure and function of aquatic ecosystems (Simon 1999; Degerman, Beier, Breine, Melcher, Quataert, Rogers, Roset & Simoens 2007). However, development of a standardised fish-based ecological assessment method for European rivers will require harmonised ecological classification and metric selection schemes as basic tools. This paper describes the approach and processes developed under the EU FAME project (http://fame.boku.ac.at/) to classify European fish species into ecological guilds and to identify suitable metrics as basic tools for the development of a new standardised ecological assessment method. Correspondence: Dr Richard Noble Hull International Fisheries Institute, University of Hull, Hull HU6 7RX, UK (e-mail: [email protected]) 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd doi: 10.1111/j.1365-2400.2007.00575.x 382 R. A. A. NOBLE ET AL. The use of functional guilds in the assessment of biotic integrity Classification according to ecological and functional guilds was developed to simplify community analysis and assist in the prediction of community change (Austen, Bayley & Menzel 1994). Root (1967) defined guilds in the ecological sense as Ôa group of species that exploit the same class of environmental resources in a similar wayÕ. Evolution has determined that each fish species has characteristic tolerances or preferences for water quality, habitat and other environmental conditions. They have specific requirements for breeding, feeding and growth. However, there is considerable overlap in the requirements and traits of many species that enables aggregation into larger functional units with similar ecological characteristics. The use of functional ecological guilds within the assessments of ecological integrity is possible because of the relationships between fish community structure and the functional complexity of riverine habitat (Goldstein & Simon 1999; Welcomme, Winemiller & Cowx 2006). The functional guild concept denotes that the fish community structure is determined by the functional diversity of the aquatic habitat, in terms of habitat available and prevalent hydrological processes (similar to river continuum concept, Vannote, Minshall, Cummins, Sedell & Cushing 1980). Within this, any changes in, or disturbance to, the functionality and structure of the riverine habitat will be reflected by responses in the functional structure of the fish community. Multi-metric indices comprise a number of metrics measuring specific aspects from a range of guilds that are known to respond in predictable ways to anthropogenic disturbance to ecosystems. Therefore, guilds and metrics are inextricably linked, given that a metric is only a unit-specific measure of a defined group of species within a community. The discrimination of the dominant fish species that characterise communities into an appropriate guild structure that yields robust metrics within an index of biotic integrity (IBI) therefore requires good levels of ecological understanding of species ecology and behaviour. Additionally, the translation of guilds into suitable metrics also requires an understanding of the limitations of the method chosen to sample the fish community, because these methods may be selective and bias community structure and composition. Selection and measurement of appropriate ecological functions for an IBI Since the first version of the IBI in North America by Karr (1981), the IBI concept has been tested and/or adapted for use throughout the world (for review, see Miller, Leonard, Hughes, Karr, Moyle, Schrader, Thompson, Daniels, Fausch, Fitzhugh, Gammon, Halliwell, Angermeier & Orth 1988; Simon & Emery 1995; Hughes & Oberdorff 1999). The development of IBIs has focussed on the combination of metrics relating to the species composition, biological diversity, species abundance and condition (e.g. prevalence of disease, hybrids and deformities) of fish communities. In most cases, the composition, diversity and abundance measures assess the functional diversity of the community. As such, metrics relating to habitat guilds, trophic guilds and reproductive guilds have been widely used. Whilst habitat, feeding and reproductive classifications have formed the key elements of functional assessments, a number of other criteria have been included. Classifications relating to the native status of species, their life-history characteristics (longevity and migration) and tolerance to stresses of various forms (e.g. pollution) have also been used in IBIs. In this context, metrics can change with the purpose of the index. If, for example, changes due to pollution are to be detected, one would use metrics sensitive to contaminants. In each new version, the list of metrics changes more or less with the target region, country or river type. Few of these metrics were used in all versions (number of species, proportion of omnivores, presence of damages and diseases), but most were specifically adapted to the features of ichthyofauna in the different countries, regions or river types. The selection of metrics was based on different criteria, such as biological importance (primary describers of the ichthyofauna status), statistical importance (selected according to their respective loading in multivariate analyses), literature references (selected by other authors), expert judgement (describing some regional disturbances of rivers or features of specific ichthyofauna) or specific applications (sensitive to specific anthropogenic degradations). However, as yet there has been no consistent approach to metric selection for development or modification of IBIs. Classification of ecological guilds and metrics Given that classification and understanding of the ecological functioning of a target fish community is a prerequisite for the development of any IBI, the development of a standardised ecological assessment tool to aid the implementation of the WFD requires that European fish species be classified in a harmonised way. Therefore, for each of the six main ecological functions identified in previous IBIs (trophic, reproduction, habitat, migration, longevity and tolerance), 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd FUNCTIONAL ECOLOGICAL GUILDS OF FISH COMMUNITIES this paper reviews the existing classifications for the associated ecological guilds, identifies the relevant ecological hypotheses for their inclusion within an IBI and discusses the limitations that exist for each group of guilds. In addition, the applications of metrics pertaining to individual (sentinel) species that may exhibit responses to degradation, and could be useful within an ecological assessment, are discussed. Finally, given the extent of species introductions and translocations across Europe, the potential differences between alien and native species within metrics are considered. Ecological classification and metric selection Trophic guilds Fish display a wide range of feeding habits and occupy many trophic roles from detritivores to secondary carnivores. However, it is rare for fish to specialise in one particular food category throughout their entire life cycle. Furthermore, many species show ontogenetic shifts in dietary preferences and plasticity in their feeding behaviour depending upon ambient conditions. Although this variability and plasticity could severely limit the powers of IBIs, trophic guilds have been consistently used in the development of IBIs. Theoretically, any changes that affect the structure and availability of food resources and/or feeding habitats alter the trophic guild structure of a fish community. In general, perturbation to the aquatic environment will tend to impact negatively on species with specialist feeding requirements (e.g. insectivores and piscivores), but favour those with flexible or diverse feeding behaviour (e.g. omnivore). Feeding classifications have developed through a variety of approaches, including combinations of dietary constituents, modes of feeding and feeding habitat. Goldstein & Simon (1999) proposed a feeding classification for North American freshwater fishes comprising five main feeding guilds and 26 modes of feeding. This classification was too complex for European fish fauna and the ecological information available. Furthermore, within the European context, few fish species have specialist feeding habitats, and these are mainly piscivores in the sub-adult and adult life stages. This represents a potential limitation to the use of feeding guilds. Consequently, a simplification was required, in which trophic guilds were defined using dominance of food items in the diet as the assessment criterion (Table 1). Classification systems that comprise elements of dietary preference together with complementary feeding information, such as mode or habitat, have the potential to overlap with other guild classifications, especially that of habitat. The definition of benthivore is a good example, as this is not a true characterisation of diet and overlaps with the habitat guild. The definition in Table 1 categorises benthivore as Ôconsisting of a high proportionÕ of benthic organisms. As such, it uses both a habitat-related definition, but also to some extent a food type definition. It is recognised that some benthivores may also, technically, be placed into one of the other categories, e.g. omnivore, if only food type is used in the definition. Consequently, the benthivore category also covers by the guild describing habitat preference. Further confusion arises between true benthivore habits and detrital feeding habits. Benthivore should be limited to molluscs and macroinvertebrates. Detrital feeders often include a range of smaller organisms in their diet. Ideally, in a classification based on the composition of the diet, when identifying species with narrow trophic niches from generalist species with wide niches, the benthivore Table 1. Proposed trophic guild classification for European fishes Guild Planktivores Herbivores Detritivores Omnivores Insectivores/ invertivores Benthivores Piscivores Parasite Adult diet Physiology/comment High proportion of zooplankton and/or phytoplankton High proportion of plant material High proportion of detritus Diet ÔgeneralistÕ, including a wide range of flora and fauna High proportion of invertebrates/insects Fine gill-rakers, elongated pharyngeal teeth, no stomach, and elongated undifferentiated intestine Terminal/sub-terminal mouth, bony slashing jaw, long digestive tract Digestive tract simple/unspecialised High proportion of benthic organisms Ventro-terminal, often highly protractile mouth. File-like teeth to comb and sort small organisms Restricted to obligate piscivores Consists of >75% fish Parasitic feeding mode Terminal/supra-terminal mouth, feed in the whole of the water column 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd 383 384 R. A. A. NOBLE ET AL. category would be omitted. However, given the limited trophic data for some species, the benthivore category is often retained in classification schemes. The same problems hold true for other categories. Difficulties in classification also arise with fish species that change their trophic status over the course of their lives, which link to ontogenetic niche shifts, change in habitat preference/occupancy and changes in diet based on availability and food partitioning. Data regarding ontogenetic shifts in diet are too variable and uncertain to be meaningful for inclusion in a standardised system for broad-scale IBI development. Consequently, classification must be limited to the trophic guild of adults, the life stage for which most data are available. Although this is a limitation, it remains the best option until better definitions of trophic guilds of each life-history stage are available. Finally, once provisionally classified, many European freshwater fish species do not fall into discrete categories, but also are not true omnivores. This is most prevalent for species that exhibit distinct trophic shifts or ranges of potential trophic status. In these circumstances, the species can be classified into joint groups, e.g. insectivore/piscivore. Such joint classifications are necessary to separate the ÔobligateÕ piscivores (e.g. Esox lucius L.) from species whose diet may include a high proportion of fish but do not solely rely on a piscivorous diet [e.g. salmonids, Anguilla anguilla (L.), Perca fluviatilis L.]. This is necessary because IBIs often use omnivore and piscivore metrics. Furthermore, species such as eel and trout switch between insectivorous and piscivorous diets under certain conditions and this may mask the response of metrics to degradation. Within an IBI, the species classification should not change under different ecological conditions (either natural or due to perturbation). Again, this type of classification is required to separate the species within specific feeding guilds from those that have wide dietary ranges. Reproductive guilds Fish exhibit diverse forms of reproduction, with species showing different spawning behaviour and using diverse spawning habitats. Consequently, reproductive guild classifications have been used within fishbased IBIs to assess changes in the structure of communities, linked to changes in the availability of different types of habitat. In many assessment methods, the lithophilic guild (gravel-spawners) and phytophilic guild (vegetation spawners) are used as measures of the reproductive structure of fish communities. The theory behind using these guilds is that as levels of degradation increase the availability and suitability of specific niches or spawning substrata are reduced, having a knock-on effect on the reproduction of species with specific spawning requirements, e.g. the loss or compaction of gravels results in a reduction in the contribution of lithophils to the community. Balon (1975) classified fish into 33 reproductive groups based on ontogeny, spawning behaviour and place of egg deposition. This classification scheme was considered inappropriate for the development of guilds for ecological assessment because many of the categories are for marine or tropical species. Also, Simon (1999) considered that assigning species to the correct reproductive guild is problematic because of paucity of behavioural and early ontogenetic data, although this is not necessarily true for European fishes, the reproductive ecologies of which are generally well known. For the purposes of developing a typology for European riverine fishes, a simplified system of reproductive guilds was developed (Table 2), based on the classification proposed by Balon (1975) and modified by Table 2. Proposed system of classification of reproductive guilds Guild Spawning habitat Lithophils Phytophils Phytolithophils Psammophils Ostracophils Pelagophils Lithopelagophils Clean gravel, rocks, stones, rubble or pebbles Plants, leaf and roots of live or dead vegetation Submerged plants, if available, or on other submerged items Roots or grass above a sandy bottom or on the sand itself Shells of bivalve molluscs Pelagic zone Rocks and gravels Ariadnophlis Speleophils Viviparous Polyphils Specialised nest building species Interstitial spaces and crevices NA Non-specialised spawners no preferred habitat Comment Includes redd cutters Pelagic embryos/larvae. Eggs initially adhesive but soon become buoyant May include some level of parental care Live bearers No specialised behaviour 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd FUNCTIONAL ECOLOGICAL GUILDS OF FISH COMMUNITIES Chadwick (1976), Mahon (1984), Bruton & Merron (1990), Oberdorff & Hughes (1992), Boet, Chessel, Hugueny, Oberdorff, Pont & Porcher (1999) and Cowx (2001). The classification was primarily based on preferred spawning habitat as this fits well with the requirements and structure of IBIs. A range of assessment metrics (Table 2) were identified that relate to reproductive guilds, including both reproductive habitat and specialised behaviour. Ariadnophilic, speleophilic, viviporous and polyphilic were included to the basic Ôreproductive habitatÕ guild classification to account for the slightly more specialised (or non-specialised in the case of polyphils) behaviour of certain European species (Table 2). Habitat guilds Each fish species has optimal habitat requirements, which result in changes in community structure along the upstream–downstream gradient of a river. These habitat requirements have long been recognised and used to classify different zones in a river (Huet 1959; Hawkes 1975) inhabited by different fish species with similar habitat preferences. It is widely acknowledged that the size, vitality and spatial distribution of species are dependent on the quantity and quality of their habitat (Karr 1991). Fish have been classified according to habitat use by numerous authors (e.g. Schlosser 1982; Leonard & Orth 1986; Bain, Finn & Booke 1988; Lobb & Orth 1991). However, many assessments of habitat preference are limited to the regions in which they were developed and are too specific for general use within an IBI. The habitat assessment within an IBI is generally designed to assess the morphological condition and hydrological functioning of the river. As such, classification schemes for habitat preference generally consider flow preferences (e.g. Schiemer & Waidbacher 1992; Aarts & Nienhuis 2003). The classifications developed include assessment of the rheophilic, eurytopic or limnophilic preferences, together with subgroups based on migration and spawning and juvenile habitat (in terms of backwaters) requirements. Aarts & Nienhuis (2003) discriminated six categories based on these two elements, therefore doubling up with other classification criteria, such as reproductive and migration guilds. Ideally, a guild system used within an IBI would not have guilds described by different ecological functions. Therefore, the habitat guild structure proposed uses only three groups: rheophilic (all freshwater life stages confined to lotic waters) eurytopic (all life stages can occur in both lentic and lotic waters) and limnophilic (all life stages confined to lentic waters). Further combinations of guilds within IBIs can be achieved by computing metrics from a combination of guilds. In most IBIs, metrics relating to the abundance or diversity of limnophilic and rheophilic species are used as a measure of the effects of impoundment and channelisation of rivers, which alter both the magnitude and variability of flow. Channelised and impounded rivers usually result in reductions in water velocity, which favours limnophilic/eurytopic species over those with rheophilic preferences. Alternatively, where a lowland river channel has been channelised and/or isolated from its floodplain by flood defence works, the abundance and number of limnophilic/ eurytopic species will be reduced. This is especially important for the eurytopic species that rely on secondary channels and floodplain water bodies for reproduction. An additional two-group classification based on feeding habitat, either water column (prefer to live and feed in the water column, usually do not go to the bottom to search for food) or benthic (prefer to live on or near the bottom, from where they take food, and usually do not go to the surface for feeding purpose), was suggested by Karr (1981). He used darter species to represent those sensitive to degradation of benthic habitats, and sunfish as those sensitive to changes in water column habitats. However, this guild may prove redundant because of the complimentary nature of the classification (i.e. a species can only be one or the other), the overlaps with trophic guilds and the problem of vertical scale dependent upon river zone. The vertical scale in shallow upland rivers is much less than that of deeper, lowland rivers, and, consequently, true benthic or water column species may only occur in the deeper lowland sections where the vertical spatial scale allows differentiation. Life-history strategies: migration guilds Ecological guilds for residency/migratory life histories are important because absence of migratory species, where they once existed, suggests a bottleneck at one or all stages of the life cycle, possibly caused by environmental perturbation or obstruction to movement. Migratory species, therefore, give potential for an assessment of the condition of a river system in terms of connectivity (Schmutz & Jungwirth 1999). However, despite the importance of connectivity (both longitudinal and lateral) to the functioning of fish communities, few IBIs have attempted to integrate metrics based on the assessment of migration guilds. Migratory behaviour of fish in rivers can be divided 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd 385 386 R. A. A. NOBLE ET AL. into two major types: potadromy and diadromy, the former referring to that occurring entirely within the inland waters of a river system (Northcote 1999), and the latter to that taking place across a transition zone between fresh and marine waters (McDowall 1997). Diadromy can be further divided into three subcategories: Anadromy (running up rivers) refers to fishes that live as older juveniles and sub-adults in the sea but at maturity migrate up rivers to spawn, e.g. Atlantic salmon, Salmo salar L. Catadromy (running down rivers) refers to fishes that live all their early life in fresh water – feeding and growing – but at maturity migrate downstream to spawn in the sea, e.g. anguillid eels. Amphidromy (running between rivers and the ocean) refers to fishes that spend appreciable parts of their life in both fresh and sea waters, feeding and growing in both, and whose migrations seem to have no direct relationship to reproduction (McDowall 1997). Assessment of the species that exhibit these different migration strategies can give valuable information to assessments of the ecological condition of rivers. However, categorisation of migration guilds is not as distinct as it first appears, and some species, e.g. Salmo trutta L., exhibit a range of migratory traits having both iteroparous and semelparous life histories. These complexities in migratory behaviour give rise to problems in standardised classification schemes as a species may exhibit different migratory behaviours in different river types. Four main classes are proposed based on migration strategy and scale; short, intermediate, long-anadromous and long-catadromous. Short and intermediate migration classes cover potadromy over different spatial scales, whilst long migration categories cover all anadromous and catadromous migration. Short migrations cover species that only moved within a particular river zone, whereas intermediate migration covers species with potadromous migrations between river zones (i.e. within river migration on a larger spatial scale, e.g. barbel, Barbus barbus (L.), and bream, Abramis brama (L.). However, for some euryhaline species [such as shads, Alosa alosa (L.) and Alosa fallax (Lacepède)] the migrations are only over an intermediate spatial scale, but are anadromous. Therefore, a fifth class, intermediate anadromous, was designated for these species. The scheme above relates to longitudinal migration. However, many species exhibit lateral migration behaviour between main river channels, side channels and floodplain waters, especially in lowland rivers (Aarts & Nienhuis 2003). The status of these species gives an indication of the morphological condition of the river channel and its connectivity with the active floodplain (Welcomme 1979; Grift, Buijse, Breteler, Van Densen, Machiels & Backx 2001; Aarts, Van Den Brink & Nienhuis 2004). Therefore, any scheme to classify migration should reflect the overall connectivity of a river system and include scope for classification of lateral migration requirements of floodplain species. Lateral migration requirements are, however, difficult to assess for many species, given a lack of understanding and the impacted nature of many European floodplains. For the purposes of an IBI approach, the use of metrics concerning the status of limnophilic and eurytopic species in river systems, species requiring lotic floodplain waters for some or all of their life cycle, is considered adequate for assessing lateral connectivity. Life history: longevity and maturation guilds Many IBIs have used classifications relating to longlived species to assess the ecological status of fish communities. These measures are designed to give some assessment of different life-history strategies (e.g. K and r strategies, Pianka 1970). Karr (1981) proposed that the status of long-lived species could integrate disturbances to the aquatic environment over multiple years. Additionally, the absence or low abundance of species with different life-history patterns may indicate different types of disturbance, or provide evidence of either acute or chronic environmental disturbance. However, classification of longevity, as a coarse measure of life history, is variable across Europe, and needs harmonisation. A longevity scheme comprising short-lived (typically < 5 yr), intermediate (5– 15 yr) and long-lived species (>15 yr) gives scope for the development of metrics for ecological assessment. In addition, within this scheme the early/late maturation of long-lived species can be formalised as 625% of the life span, giving some measure of life-history strategy. However, the longevity and age at maturation of each species can also be a reflection of the geographical location of the population within its natural range, the stability of the habitat and the optimal/sub-optimal nature of the habitat. Some species have great plasticity and can adapt their life histories to survive under different conditions (e.g. Cowx 1990). Furthermore, longevity is highly correlated with maximum size reached (Pauly 1980). Therefore, ultimately the applicability of these types of classifications to ecological assessments may be limited. 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd FUNCTIONAL ECOLOGICAL GUILDS OF FISH COMMUNITIES Tolerance capacity guilds Assessment of the tolerance of species to anthropogenic pressures has commonly been used in ecological assessment. The theory behind the use of tolerance guilds is intolerant species will be present/thrive in good conditions but absent under disturbed conditions, where tolerant species will dominate/thrive. Classifications based on the tolerance or intolerance of species to perturbations in water quality (eutrophication and/or acidification) (e.g. Alabaster & Lloyd 1982) and habitat have been used in previous IBIs developed for Europe. Breine, Simoens, Goethals, Quataert, Ercken, Van Liefferinghe & Belpaire (2004) presented a scheme for water bodies in Flanders scoring tolerance to water quality and habitat degradation on a five-point scale, with 1 being the most tolerant and 5 the most intolerant. They then calculated an overall tolerance score for each species as an average of the values assigned for tolerance to individual pressures. Oberdorff, Pont, Hugueny & Porcher (2002) empirically defined tolerant species for an IBI for French rivers using indices of water quality and habitat flexibility, as defined by Verneaux (1981) and Grandmottet (1983) respectively. Despite both schemes presenting individual scores for the species flexibility, Oberdorff et al. (2002) and Aarts & Nienhuis (2003) concluded that ultimately classification was restricted to three groups; tolerant, intermediate and intolerant. It is probable that, despite published schemes and recent research, lack of understanding of ÔtoleranceÕ, especially that of Ôoverall toleranceÕ, restricts classification to only coarse interpretations of tolerant, intolerant and intermediate. Despite the apparent logic for assessing tolerance, the value and validity of the use of tolerance guilds has not been evaluated. This is especially true where tolerance is classified based on apparent flexibility in ecological requirements. Where ÔtoleranceÕ is based on ecological flexibility, suitable habitat and trophic guild classifications could make ÔtoleranceÕ guilds redundant. Furthermore, the apparent tolerance of a certain species to a particular variable will depend upon the location of the species within its geographical range: a species in sub-optimal conditions on the edge of its range is likely to be more sensitive to an additional stressor than it would be under optimal conditions. In addition, the use of Ôoverall toleranceÕ, a simple combination of tolerances to specific pressures, may be an invalid classification given that the mechanisms of response of different species to different pressures will be variable. Furthermore, the sensitivity of species to specific pressures is complex under natural condi- tions, given that in many cases both habitat and water quality degradation are concurrent. Despite these problems with tolerance classification, an overall tolerance guild was included, based on classifications of tolerance to eutrophication, acidification and habitat degradation. Type-specific sensitive species: Ôsentinel Õ species The classification of high, good and moderate ecological status, as prescribed in the text of the WFD, takes into account the presence, abundance and population structure of Ôtype-specific sensitive speciesÕ. As such, any method developed to assess ecological status for the WFD should consider the population structure of type-specific sensitive species. This would require definition of Ôtype-specificÕ and ÔsensitiveÕ. As a simpler alternative, a number of species can be identified as ÔsentinelÕ species, i.e. those species that are deemed to be indicative of a particular river zone, but also those that will provide information on ecological status. Consequently, the sentinel species were defined as those that are relatively common within the specific river type and for which electric fishing is not size selective. In Western Europe, the key species are generally dominant and associated species for river reaches according to the Huet zonation scheme (Huet 1959). For these key species, information regarding recruitment and population structure should be assessed. As such, a range of metrics variants could be defined and tested for a number of life stages or age classes of these sentinel species. With detailed ecological knowledge, these life stages could then be classified into different ecological guilds. However, ecological information pertaining to ontogenetic shifts in ecology is often limited. Therefore, for sentinel species the easiest approach was to develop metrics for youngof-the year individuals that would provide an indication of the levels of recruitment within the population. Fish species distribution, residency and historical status of fish species Some IBIs have used metrics relating to the historical status of fish species to derive fish community typologies and define reference conditions for ÔpristineÕ ecological status (Schmutz, Kaufmann, Vogel, Jungwirth & Muhar 2000). In these cases, it has been argued that the development of an IBI requires clear definition of fish species that actually reflect ambient environmental conditions based on natural residency. This classification is important because species have now been widely introduced (e.g. rainbow trout), 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd 387 388 R. A. A. NOBLE ET AL. translocated or have become extinct in many regions (e.g. burbot, Lota lota (L)) in the UK; Welcomme 1992; Cowx 1997). Introductions and extinctions of species reflect major changes to community compositions and pose significant problems for rehabilitation. This is especially true where species introductions can have serious implications for the resident fish community. In such cases, introductions and stocking can alter the structure of the community, away from a reference condition that does not include the alien species, to a state that is not easy to rectify. However, the impacts of species introductions on the ecological status of rivers are not dealt with explicitly by the WFD. Consequently, due accord needs to be given to the residency status of species and the role they take in establishing reference conditions and assessing status. Given that alien species also have characteristic ecological traits and are subject to the same environmental pressures, they may respond to degradation in the same way as the native community. However, many introduced species are generally tolerant, which may limit their response to degradation pressures. Moreover, introduced species often persist at low densities until the environment changes, after which they can replace more specialised native species and become a pest. Therefore, the development of new methods should test metrics for both native and alien species. In this case, each metric, defined for each ecological guild, has further variants relating to whether all species or only native species are considered within each guild. The residency status of each fish species in each European drainage basin was compiled. Species were classified as native, introduced (translocated from within Europe), introduced to Europe, or endemic [to country, specific eco-region (as defined by Illies 1978) or river basin]. Where known, the date of any introduction was recorded. Any significant translocations of species between water bodies within each country were also recorded. It should be noted that in some regions, despite knowing a species was alien, the period that had elapsed since the introduction (many hundreds of years in some cases) meant that some alien species were deemed to be naturalised on a national level (e.g. common carp, Cyprinus carpio L., in Western Europe). As such, many ÔalienÕ species that have become naturalised over a long period of time are regarded as ÔnativeÕ and managed accordingly. However, to allow the metrics relating to alien and native species to be tested, if a species was not historically present in the river basin concerned it was considered an alien. This classification was expanded into a river basin/river region-specific scheme to allow the development of ichthyo-graphical regions within Europe (Reyjol, Hugueny, Pont, Bianco, Beier, Caiola, Casals, Cowx, Economou, Ferreira, Haidvogl, Noble, De Sostoa, Vigneron & Virbickas 2007) for use in the development of standardised assessment methods (Pont, Hugueny & Rogers 2007). Conclusions Ecological guilds Even with a simplified ecological classification scheme, lack of understanding of the ecological functions and requirements of species limits their accurate classification into guilds. This lack of ecological knowledge results in a high proportion of European freshwater fish species being unclassified (Table 3). Overall, only 123 of the 236 species recorded in the countries covered by the FAME project could be classified in all of the ecological functions; trophic, reproductive, habitat, migration, tolerance and longevity. Furthermore, when considering the key ecological functions of trophic, habitat and reproductive traits only 155 of the 236 species could be classified. Twenty-nine species had no ecological trait information. The functions with the lowest rate of classification were tolerance and migration. This probably highlights a lack of understanding of potadromous migration behaviour and limited understanding of the tolerance of species to perturbation. Gaps in ecological information and classification potentially pose serious problems to ecological assessments, especially if a marked proportion of a community is excluded from the assessment because they are not classified into guilds. These gaps in knowledge need to be addressed to strengthen the basic foundations of guild-based ecological assessment methods. Furthermore, gaps in knowledge for key ecological functions need to be addressed. Classification of migration behaviours can form the basis of assessments of ecological connectivity within river systems. However, it is apparent that the migration patterns and requirements of many potamodromous species are not well understood. This is probably due to the widespread impoundment of European rivers, which has occurred for many centuries. The lack of natural, un-degraded systems with free migratory access, limits interpretation of natural migration patterns. The lack of knowledge is especially true for species assemblages in the relatively unexplored river basins of Europe, e.g. The Balkans and Aegean peninsular. Furthermore, many of these river systems exhibit very different hydrological processes, e.g. intermittent flow regimes, compared with rivers of Northern Europe. 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd FUNCTIONAL ECOLOGICAL GUILDS OF FISH COMMUNITIES Table 3. Summary of the numbers of European freshwater fish species classified into the main guilds of each ecological function and the number of species that remain unclassified Ecological function Trophic Reproductive Habitat Feeding Migration Tolerance Longevity Overall Main guilds Insectivore/Invertivore Benthivore Omnivore Unclassified Lithophils Phytophils Phytolithophils Unclassified Rheophilic Eurytopic Limnophilic Unclassified Benthic Water column Unclassified Long Migratory Anadromous Long Migratory Catadromous Intermediate distance migrations (Potadromy) Unclassified Tolerant Intolerant Intermediate tolerance Unclassified Long-lived Intermediate life span Short-lived Unclassified Total number of species Classified all functions Classified key functions (trophic, habitat, reproduction) No ecological data Number of species/taxa 31 36 52 59 75 36 19 59 69 35 79 53 97 73 65 16 2 39 70 34 33 89 80 58 61 57 60 236 123 155 29 Classification based on expert panel assessment of published and grey literature. There is potential for these different hydrological regimes to translate into distinct functional ecologies and life histories for the endemic fauna of Southern Europe (see Magalhães, Beja, Canas & CollaresPereira 2002). As such, a simplified guild structure developed primarily for Northern Europe may not be suitable for southern species assemblages. However, for many of these endemic species and species assemblages, a lack of ecological data limits an assessment of the suitability of this simple guild structure. Furthermore, in many cases the taxonomic classification of these species assemblages is still debatable. Assessment of some ecological functions highlighted great plasticity within populations and between populations across their range. This is especially true with feeding guilds. Ideally, feeding guilds should be omitted from ecological assessments in their current format (Welcomme et al. 2006). Any development of feeding guilds needs to assess the plasticity in dietary habits of the species rather than purely the predominant food type. Furthermore, assessments of tolerance, particularly Ôglobal toleranceÕ, are vague and should be replaced by more suitable assessments of their plasticity in ecological functions. However, until additional ecological knowledge is available, the designation of tolerant and intolerant species will provide reasonable classification of species with known reaction to human pressure, but for which the mechanisms of reaction are not fully understood. Metric selection Review of the metrics previously used in North America and Europe suggest they assess the same aspect of a functional community, but measure them in different ways. Consequently, three criteria must be used during the selection process: ecological suitability, statistical robustness and methodological criteria. For example, the metric Ôbenthic speciesÕ can be used to assess one functional aspect of the community and its changing status with degradation, but there are many ways of measuring it: number of benthic species present, proportion of benthic species present, number of individuals, proportion of individuals, biomass or proportion of biomass of benthic species. The analytical technique used to measure the metric must be suitable to the requirements of the fish-based index, the structure of the community being studied, the ability to define a reference condition and the ease of classification and scoring of the metric within the observed range of values. This is a matter of ecological and statistical sensitivity of the metric chosen. The measure used must be sensitive enough to detect changes in the feature or function of the community being assessed. Overlaid on these criteria are criteria developed from an understanding of sampling and data limitations. Selection of metrics must therefore be justified at three levels and satisfy a range of criteria at each level. Any metric chosen must ultimately be backed up by: • ecological reasons for choosing the metric, a hypothesis for the reaction of the metric to degradation; • statistical reason for the measurement used (ability to categorise and score the range of values observed against a valid reference condition); • understanding of the limitations of the sampling procedure used to assess the metric. In conclusion, the main criterion for selecting the most relevant metrics is that a candidate metric (in 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd 389 390 R. A. A. NOBLE ET AL. Figure 1. Schematic of potential metrics belonging to the species composition category that should be tested in the development of an index of biotic integrity. For each guild all potential metrics variants are indicated. * indicates that Ôspecialised speciesÕ excludes lithophilic and phytophilic species. terms of a guild or ecological function) should be proposed in relation to the expected variation with degradation. Once this list of candidate metrics is identified, all potential variants of the metrics should be tested rather than merely choosing one or a couple of options. An example of the matrix of potential metrics relating to species composition is presented in Figure 1. In the matrix, the guilds identified are those selected from a hypothesis for their response to degradation and the multitude of unit measurements are all variants that should be tested statistically. Acknowledgements Many thanks are due to all the members of the FAME consortium who contributed to the harmonised classification, particularly N. Roset, J. de Leuw, E. Winter, R. Haberbosch, J. Breine, E. Degerman J. Oliveira, M. Lapinska, T. Virbickis, A. Melcher, N. Caiola and R. Barbieri. We would like to thank the European Commission for funding the FAME project (EVK1CT-2001-00094). References Aarts B.G.W. & Nienhuis P.H. (2003) Fish zonations and guilds as the basis for assessment of ecological integrity of large rivers. Hydrobiologia 500, 157–178. Aarts B.G., Van Den Brink F.W.B. & Nienhuis P.H. (2004) Habitat loss as the main cause of the slow recovery of fish faunas of regulated large rivers in Europe: the transversal floodplain gradient. River Research and Applications 20, 3– 23. Alabaster J.S. & Lloyd R. (1982) Water Quality Criteria for Freshwater Fish, 2nd edn. Food and Agriculture Organisation of the United Nations, London: Butterworths, 361 pp. 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd FUNCTIONAL ECOLOGICAL GUILDS OF FISH COMMUNITIES Austen D.J., Bayley P.B. & Menzel B.W. (1994) Importance of the guild concept to fisheries research and management. Fisheries 19, 12–20. Bain M.B., Finn J.T. & Booke H.E. (1988) Streamflow regulation and fish community structure. Ecology 69, 382– 392. Balon E.K. (1975) Reproductive guilds of fishes – a proposal and definition. Journal of the Fisheries Research Board of Canada 32, 821–864. Boet P., Chessel D., Hugueny B., Oberdorff T., Pont & Porcher J.P. (1999) Rapport final de la Phase II du programme national (Indice Poisson). Paris, France: Conseil Superieur de la Peche, Direction Generale, 60 pp. Breine J., Simoens I., Goethals P., Quataert P., Ercken D., Van Liefferinghe C. & Belpaire C. (2004) A fish-based index of biotic integrity for upstream brooks in Flanders (Belgium). Hydrobiologia 522, 133–148. Bruton M.N. & Merron G.S. (1990) The proportion of different eco-ethological sections of reproductive guilds of fishes in some African inland waters. Environmental Biology of Fishes 28, 179–187. Chadwick E.M.P. (1976) Ecological fish production in a small Precambrian shield lake. Environmental Biology of Fishes 1, 13–60. Cowx I.G. (1990) Growth and reproduction tactics of roach, Rutilus rutilus (L.), and dace, Leuciscus leuciscus (L.), populations in the Rives Exe and Culm, England. Polskie Archiwum Hydrobiologii 37, 195–210. Cowx I.G. (1997) Introduction of fish species into European freshwaters: economic success or ecological disasters? Bulletin Français de la Peˆche et de la Pisciculture 344–345, 57–78. Cowx I.G. (2001) Factors Influencing Coarse Fish Populations in Rivers: A Literature Review, R & D Publication 18. Bristol, UK: Environment Agency, 146 pp. Degerman E., Beier U., Breine J., Melcher A., Quataert P., Rogers C., Roset N. & Simoens I. (2007) Classification and assessment of degradation in European running waters. Fisheries Management and Ecology 14, 417–426. EU Water Framework Directive. (2000) Directive 2000/60/ EC of the European Parliament and the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities (22.12.2000) L 327, 1). Goldstein R.M. & Simon T.P. (1999) Toward a united definition of guild structure for feeding ecology of North American freshwater fishes. In: T.P. Simon (ed.) Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities. Boca Raton, FL: CRC Press, pp. 123–220. Grandmottet J.P. (1983) Principales exigencies de téléostéens dulcicoles vis-à-vis de lÕhabitat aquatique. Annales Scientifique de Universite Besançon 4, 3–32 (in French). Grift R.E., Buijse A.D., Breteler J.G.P.K., Van Densen W.L.T., Machiels M.A.M. & Backx J.J.G.M. (2001) Migration of bream between the main channel and floodplain lakes along the lower River Rhine during the connection phase. Journal of Fish Biology 59, 1033–1055. Hawkes H.A. (1975) River zonation and classification. In: B. A. Whitton (ed.) River Ecology. Oxford: Blackwell Scientific Publications, pp. 312–374. Huet M. (1959) Profiles and biology of Western European streams as related to fish management. Transactions of the American Fisheries Society 88, 155–163. Hughes R.M. & Oberdorff T. (1999) Aplications of IBI concepts and metrics to waters outside the United States and Canada. In: T.P. Simon (ed.) Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities. Boca Raton, FL: CRC Press, pp. 79–93. Illies J. (1978). Limnofauna Europaea. Stuttgart: Gustav Fischer Verlag, 241 pp. Karr J.R. (1981) Assessment of biotic integrity using fish communities. Fisheries 6, 21–27. Karr J.R. (1991) Biological integrity: a long-neglected aspect of water resource management. Ecological Applications 1, 66–84. Leonard P.M. & Orth D.J. (1986) Application and testing of an index of biotic integrity in small, coolwater streams. Transactions of the American Fisheries Society 115, 401– 414. Lobb M.D. & Orth D.J. (1991) Habitat use by an assemblage of fish in a large warmwater stream. Transactions of the American Fisheries Society 120, 65–78. Magalhães M.F., Beja P., Canas C. & Collares-Pereira M.J. (2002) Functional heterogeneity of dry-season fish refugia across a Mediterranean catchment: the role of habitat and predation. Freshwater Biology 47, 1919–1934. Mahon R. (1984) Divergent structure in fish taxocenes of North Temperate streams. Canadian Journal of Fisheries and Aquatic Sciences 41, 330–350. McDowall R.M. (1997) The evolution of diadromy in fishes (revisited) and its place in phylogenetic analysis. Reviews in Fish Biology and Fisheries 7, 443–462. Miller D.L., Leonard P.M., Hughes R.M., Karr J.R., Moyle P.B., Schrader L.H., Thompson B.A., Daniels R.A., Fausch K.D., Fitzhugh G.A., Gammon J.R., Halliwell D.B., Angermeier P.L. & Orth D.J. (1988) Regional applications of an index of biotic integrity for use in water resource management. Fisheries 13, 12–20. Northcote T.G. (1999) Migratory behaviour of fish and its significance to movement through riverine fish passage facilities. In: M. Jungwirth, S. Schmutz & S. Weiss (eds) Fish Migration and Fish Bypasses. Oxford: Fishing News Books, Blackwell Science, pp. 3–18. Oberdorff T. & Hughes R.M. (1992) Modification of an index of biotic integrity based on fish assemblages to 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd 391 392 R. A. A. NOBLE ET AL. characterize rivers of the Seine Basin, France. Hydrobiologia 228, 117–130. Oberdorff T., Pont D., Hugueny B. & Porcher J.P. (2002) Development and validation of a fish-based index (FBI) for the assessment of ‘‘river health’’ in France. Freshwater Biology 47, 1720–1734. Pauly D. (1980) On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. Journal Conseil intermationale Exploration de la Mer 39, 175–192. Pianka E.R. (1970) On ‘‘r’’ and ‘‘k’’ selection. American Naturalist 104, 592–597. Pont D., Hugueny B. & Rogers C. (2007) Development of a fish-based index for the assessment of river health in Europe: the European Fish Index. Fisheries Management and Ecology 14, 427–439. Reyjol Y., Hugueny B., Pont D., Bianco P.G., Beier U., Caiola N., Casals F., Cowx I., Economou A., Ferreira T., Haidvogl G., Noble R., De Sostoa A., Vigneron T. & Virbickas T. (2007) Patterns in species richness and endemism of European freshwater fish. Global Ecology and Biogeography 16, 65–75. Root R.B. (1967) The niche exploitation pattern of the bluegray gnatcatcher. Ecological Monographs 37, 317–350. Schiemer F. & Waidbacher H. (1992) Strategies for conservation of a Danubian fish fauna. In: P.J. Bonn, P. Calow & G.E. Petts (eds) River Conservation and Management. Chichester: John Wiley and Sons, pp. 363–382. Schlosser I.J. (1982) Fish community structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52, 395–414. Schmutz S. & Jungwirth M. (1999) Fish as indicators of large river connectivity: the Danube and its tributaries. Archiv fur Hydrobiologie Suppl. 115, 329–348. Schmutz S., Kaufmann M., Vogel B., Jungwirth M. & Muhar S. (2000) A Multi-level concept for fish-based, river-type-specific assessment of ecological integrity. Hydrobiologia 422/423 279–289. Simon T.P. (ed.) (1999) Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities. Washington, DC: CRC Press, 671 pp. Simon T.P. & Emery E.B. (1995) Modification and assessment of an index of biotic integrity to quantify water resource quality in Great Rivers. Regulated Rivers: Research and Management 11, 283–298. Vannote R.L., Minshall G.W., Cummins K.W., Sedell J.R. & Cushing C.E. (1980) The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37, 130–137. Verneaux J. (1981) Les poissons et la qualité des cours dÕeau. Annales Scientifique de Universite Besançon 4, 33–41 (In French). Welcomme R.L. (1979) Fisheries Ecology of Floodplain Rivers. London: Longman, 317 pp. Welcomme R.L. (1992) The conservation and environmental management of fisheries in inland and coastal waters. Netherlands Journal of Zoology 42, 176–189. Welcomme R.L., Winemiller K.O. & Cowx I.G. (2006) Fish environmental guilds as a tool for assessment of ecological condition of rivers. Rivers Research and Applications 22, 377–396. 2007 The Authors. Journal compilation 2007 Blackwell Publishing Ltd