* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Short-term heart rate variability during a cognitive challenge in
Saturated fat and cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Coronary artery disease wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
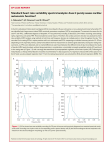
# Age and Ageing 2002; 31: 131–135 2002, British Geriatrics Society Short-term heart rate variability during a cognitive challenge in young and older adults R OBERT W OOD, B RIAN M ARAJ1 , C. M ATTHEW L EE, R AFAEL R EYES Louisiana State University, Department of Kinesiology, Baton Rouge, Louisiana, USA 1 University of Alberta, Department of Physical Education and Recreation, Alber ta, Calgary, Canada Address correspondence to: R. Wood. 112 Long Field House, Baton Rouge, L A 70803, USA. Fax: (q1) 225 578 3680. Email: [email protected] Abstract Background: attention-demanding tasks cause changes in the autonomic modulation of cardiac function. Heart rate variability, an index of autonomic modulation of heart rate, decreases with age. Objective: to examine heart rate variability in elderly and young participants at rest and during an attentiondemanding task. Methods: we assessed 16 old participants (ages 72–91) and 16 college-age (ages 20–25) participants for shortterm (5 min) heart rate variability at rest and during a simple-reaction time task. We report heart rate variability as the standard deviation of all interbeat intervals, and as the relative contribution of changes occurring at low- and high-frequencies. Results: there were no group differences in resting heart rate. A 262 mixed model ANOVA suggested a main effect of age on standard deviation of all interbeat intervals (P-0.05) which was significantly lower for the older group than their younger counterparts. There was also a significant effect of the test condition on standard deviation of all interbeat intervals and spectral measures of heart rate variability (P-0.05) in that standard deviation of all interbeat intervals dropped during the simple reaction time as did high-frequencies, while normalized low frequency power increased. Conclusion: cardiac autonomic modulation during provocative stress show similar physiologic responses in young and older adults. Keywords: ageing, cardiovascular, heart, autonomic, stress Introduction Chronological age is inversely related to autonomic modulation of heart rate: older people have reduced heart rate variability (HRV) [1–3]. Frequency domain analyses reveal that the lower variance in heart rate observed in older adults is marked by global reductions across the entire power spectrum [2], suggesting that sympathetic and parasympathetic modulation of heart rate are inversely related to age, but that sympathovagal balance is not age-dependent. Although the mechanism(s) responsible for the age-related changes in autonomic control of the heart are not completely understood, considerable evidence points towards impairment at the level of the receptor or post-receptor (e.g. cyclic AMP activity) and not necessarily the autonomic nervous system itself [4, 5]. Whatever the physiological mechanisms, age-related changes in HRV are clinically important as low HRV is associated with risk of cardiovascular and all-cause death [6, 7]. Acute periods of mental effort also result in reduced HRV [8–10]. This phenomenon suggests a mechanism through which psychological stress might exacerbate cardiac rhythm disturbances. This may be of particular concern among older adults who, having low baseline HRV, appear particularly susceptible to such events. Therefore we examined the effect of cognitive effort on HRV in healthy young and elderly adults. Data from previous investigations indicate that older subjects typically present exaggerated elevations of plasma catecholamines and exaggerated parasympathetic withdrawal during stressful tasks [4, 11]. However, reports of end-organ responsiveness are equivocal, varying from exaggerated [12], to no difference in [13] 131 R. Wood et al. or, in some cases, smaller cardiovascular responses [11, 14, 15] among older adults. With respect to HRV, the few available studies are also somewhat disparate. Ferrari et al. [16] observed a lower cardiac responsiveness to graded electrical stimulation of the vagus nerve in older as compared to younger rats. Consistent with this are the recent findings of Uchino et al. [12] who reported an inverse relationship between age and parasympathetic withdrawal during psychological stress. However, Boutcher and Stocker [17] reported healthy young and old men to have similar stress-induced changes in HRV. In contrast to these previous studies, we have examined apparently healthy elderly adults (over 70). We have also examined the spectral measures of HRV to provide indices of sympathovagal balance. We hypothesized that the older participants would have low resting overall HRV, setting the stage for a blunted response to cognitive challenge. We also hypothesized that the change in sympathovagal balance during the cognitive challenge would not be age-dependent, as both sympathetic and parasympathetic modulation of the heart appear to deteriorate at a similar rate with age [2, 12]. Methods Participants Forty-two independent residents of a retirement community responded to an invitation to participate in this study. We obtained current medical records (i.e. within one year, or since the onset of any symptoms within the last year) to screen for the presence or history of diagnosed chronic heart failure, myocardial infarction, hypertension, and neurological conditions. Other exclusion criteria included the use of drugs that could affect cardiovascular function. All medications were discontinued for 24 h before data collection. Nineteen respondents met the inclusion criteria and completed the study (15 women and 4 men). The age range of this group of participants was 72–93 years. Twentyeight younger participants (ages 19–23) also responded to an invitation to participate. Nineteen were assigned to the young group to sex-match the elderly and young groups. Procedures The institutional review board of the host site approved all procedures. On arriving at the testing site, the participant provided written informed consent and completed a standard medical history questionnaire. Following the administration of these items, visual acuity was assessed using a Snellen eye chart that was read from 20 feet. The participant was allowed to use corrective lenses throughout the study. For auditory problems, we relied only on self-reporting and medical histories. 132 Electrocardiograph (ECG), pnuemotachograph, and blood pressure data were then collected during 5 min of spontaneous breathing and during 5 min of the simple reaction time task. During spontaneous breathing, the participant sat motionless in a chair, without engaging in conversation. During the reaction time task, the participant responded to visual and auditory cues by depressing a keypad device as quickly as possible. The visual stimulus was a light encased in a small circular frame that was illuminated on a panel. The auditory stimulus was a tone-generated device: as soon as subjects heard the sound, they depressed the switch. There was no choice of keys involved in this task. The participant responded to two sets of 50 auditory cues and two sets of 50 visual cues, the order of which was counterbalanced. A Biopac [18] data acquisition board was used to collect ECG and pnuemotachograph data throughout, and blood pressure was assessed twice by mercury sphygmomanometry during each 5-minute period. We report the averages of the two observations for systolic and diastolic pressure. Data analysis We collected the 5-minute ECG and pnuemotachograph data at 200 Hz and converted them to tachograms of consecutive interbeat intervals and respiratory frequency, respectively. We used the tachogram of respiratory frequency to assess mean respiratory rate. We analyzed the interbeat interval data for the standard deviation of all normal interbeat intervals (SDNN), an estimate of overall HRV which may reflect vagal modulation of the heart [19]. The tachogram was then resampled at 4.0 Hz and subjected to a fast Fourier transformation using a Hamming window. We then analyzed the squared modulation of the resultant frequency spectrum (power spectrum) for normalized low- and high-frequency power (LFnu = 0.04–0.15 Hz, and HFnu = 0.15–0.40 Hz, respectively) in accordance with established guidelines [19]. Statistical analysis We used a 262 (age group6test condition) mixedmodel ANOVA, with repeated measures on the test condition, to evaluate the dependent measures for differences according to test condition or age group, and age group by test condition interactions. The GreenhouseGeisser method of controlling for sphericity of values was employed (sphericity of values, or circularity, refers to the General Linear Modal assumption of equal variances in the context of repeated-measures designs [20]). Alpha was set at 0.05. Results The descriptive statistics for the dependent measures are presented in Table 1. Age, stress, and heart rate variability Table 1. Descriptive statistics Participants Age-range Test condition Young 20–25 years Spontaneous breathing SRT Elderly 72–91 years Spontaneous breathing SRT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Visual-SRT (msec) Auditory-SRT (msec) RR (msec) SDNN (msec) HFnu LFnu SBP (mmHg) DBP (mmHg) Respiratory Rate (breaths/min) – – 887"52.3 60.7"4.7 0.63"0.19 0.37"0.19 116.4"10.7 78.2"6.2 14.2"2.2 287.0"35.3 213.1"33.8 763"48.6b 50.6"4.6b 0.53"0.15b 0.47"0.15b 132.6"12.5b 81.4"7.1 14.6"2.8 – – 859"60.1 29.3"4.3a 0.63"0.16 0.37"0.16 131.9"13.1a 80.2"8.9 14.8"2.7 401.6"61.9a 295.8"72.2a 723"52.3b 22.9"3.7a,b 0.57"0.22b 0.43"0.22b 141.0"10.2a,b 84.4"7.9 15.2"3.3 a Main effect of age group (P-0.05). Main effect of test condition (P-0.05). Values are mean"standard deviation. SRT, simple reaction time task; RR, heart period (RR interval); SDNN, standard deviation of all normal interbeat (RR) intervals; HFnu, normalized high-frequency power [HF/(HFqLF)]; LFnu, normalized low-frequency power [LF/(HFqLF)]. b Age-related differences The ANOVA revealed (P-0.05) a main effect of age: the young participants demonstrated a higher SDNN and lower systolic blood pressure than their elderly counterparts at rest and during the reaction time task (Table 1). There were no effects of age under either test condition on heart period, spectral measures of HRV, diastolic blood pressure, or respiratory frequency. Effects of the simple reaction time task The performance of the reaction time task resulted in (P-0.05) a shortening of the heart period, an increase in systolic blood pressure, and a reduction in SDNN and HFnu (and by definition, an increase in LFnu) (Table 1) in both the young and elderly participants. The simple reaction time task did not evoke changes in diastolic blood pressure. There were no significant interactions between age and the test condition. Discussion These results are consistent with an age-related decrease in overall short-term HRV (SDNN), but no age-related differences in spectral measures [1–3]. The global reduction across all power bands may reflect a downregulation of cardiac receptor sensitivity that appears with advanced age [4, 5]. As is often the case in cross sectional studies, the assignment of group differences to the effects of age is limited. Despite screening for disease in the study sample, the possibility of undetected cardiac and metabolic disease cannot be ruled out completely, nor can the long-term effects of drug actions and interactions. However, in support of the notion that the study sample is relatively healthy, there was a lack of age-related difference in resting heart period. Heart period changes very little with age in the absence of disease (see [21] for a review). These data indicate a main effect of the simple reaction time task on HRV (SDNN and spectral measures) and systolic blood pressure. These findings suggest a withdrawal of vagal modulation of the heart and a shift towards greater sympathovagal balance (i.e. a relative increase in the proportion of power that is sympathetic in origin). Previous investigations indicate that cognitive effort reduces overall HRV in different populations [8–10, 16, 17]. The findings of the present study suggest that such an effect is also apparent among elderly subjects, but do not support the hypothesis that an interaction between age and the stressor would exist. Thus, these data extend the findings of Boutcher and Stocker to elderly subjects, and contrast with the findings of Uchino et al. [12]. The main effects of age and mental effort can be visualised in the Poincare plots shown in Figure 1, depicting heart rate data for one elderly and one young adult. These plots illustrate an intact end-organ response to mental effort in elderly participants. With respect to the spectral components of HRV, our findings are in agreement with Pagani et al. [9] who observed augmented sympathovagal balance during the cognitive challenge. Wood et al. [10] did not observe such changes, despite a decrement in overall HRV (i.e. SDNN) during the cognitive task. The lack of agreement among these investigations may reflect the controversy surrounding the use of low-frequency power to quantify sympathetic modulation of the heart. Autonomic blockade studies [22] indicate that vagal modulation of the heart can show up in the low-frequency power band. This makes it difficult to suggest that our findings are truly indicative of a shift towards greater sympathovagal 133 R. Wood et al. Poincare Plots of Heart Rate a b c d Figure 1. Poincare plots of heart rate by age and test condition. The plots in (c) represent the heart rate defined by one R–R interval (HRn) plotted against the heart rate from the next successive R–R interval (HRnq1). These plots are for one young participant (age = 21 years) and one elderly (age = 85 years) participant during the 5-minute paced breathing and SRT test conditions. Narrow or ‘torpedo’ shaped distributions of data points (as with the elderly participant, or as observed during the SRT) have been associated with greater sympathetic modulation of the heart. balance, or if the heightened LFnu is merely reflecting a greater proportion of vagal modulation occurring at a low frequency. In conclusion, these data suggest that despite the age-related differences in HRV, the central cardiovascular response to mental effort in healthy elderly adults remains intact. Clinicians are beginning to use psychological stress testing for quantifying cardiovascular risk and disease severity [22], particularly among individuals for whom traditional measures (such as graded exercise tolerance tests) are not appropriate. Thus the continued development of psychological stress testing may be of particular benefit to elderly people. To this end, it is important to characterize the cardiovascular responses in healthy and diseased older individuals, and to include in particular the quantification of HRV, as it may provide unique information on risk of arrhythmogenesis and cardiac and all-cause mortality. Key points . Ageing is associated with reduced heart rate variability. . Exposure to cognitive challenges further reduces heart rate variability. . The magnitude of change in heart rate variability during a cognitive challenge does not appear to be a function of age. 134 Acknowledgements We would like gratefully to acknowledge the St James Place Continuing Care Retirement Community in Baton Rouge, Louisiana for their co-operation in this study. References 1. Parati G, Frattola A, Di Rienzo M et al. Broadband spectral analysis of blood pressure and heart rate variability in very elderly subjects. Hypertension 1997; 30: 803–8. 2. Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J Gerontol Med Sci 1991; 46: M99–106. 3. Shannon DC, Carley DW, Benson H. Aging modulation of heart rate. Am J Physiol 1997; 253: H874–7. 4. Esler MD, Thompson JM, Kaye DM et al. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation 1995; 91: 351–8. 5. Morrow LA, Linares OA, Hill TJ et al. Age differences in the plasma clearance mechanisms for epinephrine and norepinephrine in humans. J Clin Endocrinol Metab 1987; 65: 508–11. 6. Kautzner J, Camm AJ. Clinical relevance of heart rate variability. Clin Cardiol 1997; 20: 162–8. 7. Dekker JM, Schouten EG, Klootwijk P et al. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. Am J Epidemiol 1997; 145: 899–908. Age, stress, and heart rate variability 8. deGeus EJC, vanDoornen LJP, deVisser AC, Orlebeke JF. Existing and training-induced differences in aerobic fitness: their relationship to psychological response pattern during different types of stress. Psychophysiol 1990; 27: 457–78. 9. Pagani M, Mazzuero G, Ferrari A et al. Sympathovagal interaction during mental stress. Circulation 1991; 83: 43–51. 10. Wood RH, Wood WA, Welsch MA, Avenal P. Physical activity, mental stress, and short-term heart rate variability in patients with ischemic heart disease. J Cardiopul Rehab 1998; 18: 271–6. 11. Barnes RF, Raskind M, Gumbrecht G, Halter JB. The effects of age on the plasma catecholamine response to mental stress. J Clin Endocrinol Metab 1982; 54: 64–9. 16. Ferrari AJ, Deffanchio A, Gerosa S, Mancia G. Alterations in cardiac parasympathetic function in aged rats. Am J Physiol 1991; 260: H647–9. 17. Boutcher SH, Stocker D. Cardiovascular response of young and older males to mental challenge. J Gerontol: Psycholog Sci 1996; 51B (5): P261–5. 18. Biopac Systems, Inc. ACQKNOWLEDGE 100 (data acquisition software). Santa Barbara, CA, 1994. 19. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 1996; 93: 1043–65. 12. Uchino BN, Uno D, Holt-Lunstad J, Flinders JB. Agerelated differences in cardiovascular reactivity during acute psychological stress in men and women. J Gerontol Psychol Sci Soc Sci 1999; 54: 339–46. 20. Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika 1959; 24: 95–112. 13. Gotthardt U, Schweiger U, Fahrenberg J. ACTH and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol 1995; 268: R865–73. 22. Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. J Auton Nerv Syst 1995; 51: 85–9. 21. Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 1993; 73: 413–67. 14. Gintner GG, Hollandsworth JG, Intrieri RC. Age differences in cardiovascular reactivity under active coping conditions. Psychophysiol 1986; 23: 378–85. 15. Steptoe A, Fieldman G, Evans O, Perry L. Cardiovascular risk and responsivity to mental stress: the influence of age, gender, and risk factors. J Cardiovasc Risk 1996; 3: 83–93. Received 27 April 2001; accepted in revised form 25 October 2001 135