* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The CALF (Congenital Heart Disease in Adults Lower Extremity
Survey
Document related concepts
Transcript
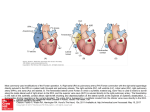
Journal of the American College of Cardiology © 2010 by the American College of Cardiology Foundation Published by Elsevier Inc. Vol. 56, No. 2, 2010 ISSN 0735-1097/$36.00 doi:10.1016/j.jacc.2010.02.048 Congenital Heart Disease The CALF (Congenital Heart Disease in Adults Lower Extremity Systemic Venous Health in Fontan Patients) Study Anne Marie Valente, MD,*† Ami B. Bhatt, MD,*† Stephen Cook, MD,‡ Michael G. Earing, MD,§ Deborah R. Gersony, MD,储 Jamil Aboulhosn, MD,¶ Alexander R. Opotowsky, MD, MPH,# George Lui, MD,储 Michelle Gurvitz, MD, MS,** Dionne Graham, PHD,* Susan M. Fernandes, PA-C,* Paul Khairy, MD, PHD,* Gary Webb, MD,# Marie Gerhard-Herman, MD,† Michael J. Landzberg, MD,*† for the AARCC (Alliance for Adult Research in Congenital Cardiology) Investigators Boston, Massachusetts; Columbus, Ohio; Milwaukee, Wisconsin; New York, New York; Los Angeles, California; Philadelphia, Pennsylvania; and Seattle, Washington Objectives The objective of this study was to document the prevalence of chronic venous insufficiency (CVI) and its associated factors in adults with Fontan physiology. Background As the population of adults with complex congenital heart disease and Fontan physiology increases, so does the occurrence of highly morbid and mortal outcomes, including heart failure and thromboembolism. The presence of abnormal peripheral hemodynamic conditions in this population and their potential contribution to adverse outcomes is not well known. The primary objective of this study was to document the prevalence of CVI in adults with Fontan physiology. Methods A total of 159 adults with Fontan physiology from 7 adult congenital heart centers were prospectively assessed for lower extremity CVI, with the assignment of clinical, etiological, anatomical, and pathophysiological classification grades, and compared with age-matched and sex-matched controls. Leg photographs were independently reassessed to confirm interobserver reliability. Results The prevalence of CVI was significantly greater in the Fontan population (60%; 95% confidence interval [CI]: 52% to 68%) compared with healthy controls (32%; 95% CI: 15% to 54%) (p ⫽ 0.008). Strikingly, the prevalence of severe CVI (clinical, etiological, anatomical, and pathophysiological grade ⱖ4) was significantly higher in the Fontan group (22%; 95% CI: 16% to 29%) versus the healthy controls (0%; 95% CI: 0% to 14%) (p ⫽ 0.005). In a multivariate analysis, several factors were independently associated with severe CVI, including increased numbers of catheterizations with groin venous access, lower extremity itching, and deep venous thrombosis. Conclusions CVI is common in adult patients with congenital heart disease with Fontan physiology. The contribution of abnormal peripheral hemodynamic conditions to comorbidities, including thromboembolism and heart failure, and interventions to improve peripheral hemodynamic conditions require further exploration. (J Am Coll Cardiol 2010;56:144–50) © 2010 by the American College of Cardiology Foundation The Fontan operation has evolved over the past 4 decades from palliation for tricuspid atresia to its wider current application for many single-ventricle circulations (1). Gradual attrition in survival in adults with Fontan physiology is related to thromboembolism, heart failure and sudden death (2), whereas loss of functional ability has largely been correlated with central hemodynamic status, alterations in neurohormonal activation, and imbalance in ventilatory function (3–7). More recently, there has been recognition of the potential impact of changes in peripheral hemodynamic conditions, From the *Department of Cardiology, Children’s Hospital Boston, Boston, Massachusetts; †Division of Cardiology, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts; ‡Ohio State University, Columbus, Ohio; §Medical College of Wisconsin, Milwaukee, Wisconsin; 储Columbia University, New York, New York; ¶University of California, Los Angeles, Los Angeles, California; #University of Pennsylvania, Philadelphia, Pennsylvania; and the **University of Washington, Seattle, Washington. This study was supported in part by the Dunlevie Fund (Boston, Massachusetts). The Alliance for Adult Research in Congenital Cardiology has received support from the Adult Congenital/Pediatric Cardiology Section of the American College of Cardiology. Manuscript received September 16, 2009; revised manuscript received February 5, 2010, accepted February 15, 2010. Valente et al. Venous Disease in Adult Fontan Patients JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 skeletal muscle metabolism, and endothelial dysfunction on the failing Fontan physiology. Endothelium-dependent circulating levels of von Willebrand factor, flow-mediated dilation, and blood flow supply to skeletal muscle with subsequent effect on exercise capacity have all been shown to be abnormal in patients with Fontan palliation (8 –11). See page 151 Chronic venous insufficiency (CVI) is a common clinical problem. In the general population, CVI is associated with a reduced quality of life, particularly in relation to pain, depression, social isolation, mobility, and physical function (12). Several pathophysiologic mechanisms appear common to the occurrence of both CVI and Fontan failure, including abnormalities in central and peripheral hemodynamic status. However, to date, CVI has not been described in patients who have undergone the Fontan procedure. Risk factors for CVI in the general population (including heredity, age [13–15], female sex, leg trauma, obesity, history of phlebitis or deep vein thrombosis [16], pregnancy [17], oral contraceptive use, and prolonged standing) and symptoms referable to CVI (including aching, heaviness, a sensation of swelling, and skin irritation) can be determined by clinical history. Inspection and palpation define physical findings to further classify CVI. CVI can be thereby identified and graded according to a well-established scale describing clinical, etiological, anatomical, and pathophysiological (CEAP) classifications (18,19). The objective of this study was to document the prevalence of CVI and its associated factors in adults with Fontan physiology. The hypothesis was that patients with Fontan physiology have chronically elevated systemic venous pressure, and this finding in the setting of nonpulsatile pulmonary blood flow may place them at increased risk for CVI. These results may further elucidate the complex relationships between central and peripheral hemodynamic conditions, as well as provide a foundation for future studies aiming to improve functional outcomes in this population. Methods Study design. This multi-institutional, cross-sectional, observational study prospectively recruited successive subjects age ⱖ18 years who had previously undergone Fontan procedures and presented in stable condition for outpatient evaluation at 1 of 7 U.S. adult congenital heart disease centers for evaluation. The protocol was developed by the core institution (Children’s Hospital Boston, Boston, Massachusetts) and was refined by the consensus of participating centers within the Alliance for Adult Research in Congenital Cardiology framework (20). Subjects were excluded if they had histories of cardiac transplantation or had major surgery or traumatic injury within the previous 10 weeks. Clinical, anatomical, and surgical data were collected at enrollment (July 2007 to May 2009) by medical record 145 review and patient interview usAbbreviations and Acronyms ing standardized forms. Control subjects consisting of hospital CEAP ⴝ clinical, employees without histories of etiological, anatomical, and congenital heart disease were pathophysiological identified and enrolled. A comCI ⴝ confidence interval parison group of subjects cared CVI ⴝ chronic venous for by the core institution who insufficiency had previously undergone tetralTOF ⴝ tetralogy of Fallot ogy of Fallot (TOF) surgical repair were also included. The protocol was approved by each center’s institutional review board, and written informed consent was obtained. Recruitment protocol. Standardized case report forms included basic demographic information, congenital cardiac anatomical diagnosis as designated by the treating center using standardized nomenclature (21), surgical procedures including type of Fontan operation, current medications, family history of venous disease, and symptoms. Laboratory values and historical imaging data that were collected within 1 year of enrollment were included. A directed physical examination was performed, including a thorough evaluation of the subjects’ lower extremities with the assignment of CEAP classification grades by the investigator at each center. The clinical signs in the affected legs are categorized into 7 classes designated C0 to C6 (Table 1) (18). Patients with CEAP grades ⱖ4 are categorized as having severe CVI, given the presence of end-organ changes from lipodermatosclerosis to ulceration (22). Each center used a digital camera to photograph the lower extremities according to a standardized protocol. The photographs were taken at a distance of 1 to 2 feet from the subject while he or she was standing in 4 distinct positions. Additional photographs were taken at close range of any ulcers, discoloration, or other skin changes. Interobserver agreement. To test agreement of CEAP classification by each center, a vascular medicine specialist (M.G.H.) blinded to the subjects’ assigned CEAP classifications reviewed 50 randomly selected subjects and determined CEAP grades on the basis of visual inspection of the photographs provided. Statistical analysis. Continuous demographic and clinical data are summarized as mean ⫾ SD or as medians and CEAP TableClassification 1 CEAP Classification Class Characteristics 0 No visible or palpable signs of venous disease 1 Telangiectasias or reticular veins 2 Varicose veins 3 Edema 4a Skin changes ascribed to venous disease (pigmentation, eczema) 4b Skin changes including lipodermatosclerosis and/or atrophie blanche 5 Skin changes as defined above with healed venous ulcer 6 Skin changes as defined above with active venous ulcer CEAP ⫽ clinical, etiological, anatomical, and pathophysiological. 146 Valente et al. Venous Disease in Adult Fontan Patients JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 Baseline of Fontan, TOF, and Healthy Control Subjects Table 2 Characteristics Baseline Characteristics of Fontan, TOF, and Healthy Control Subjects Fontan (n ⴝ 159) TOF (n ⴝ 40) p Value Healthy (n ⴝ 25) p Value 30.6 ⫾ 9.1 35.6 ⫾ 10.7 0.003 32.1 ⫾ 7.6 0.43 Age at surgical repair* (yrs) 8.3 (4.3–15.2) 5.7 (1.5–10.0) 0.007 Male sex 73 (46%) 16 (40%) 0.50 10 (40%) 0.58 25.7 ⫾ 5.9 0.14 23.1 ⫾ 2.5 0.10 Characteristic Age at enrollment (yrs) Body mass index (kg/m2) Cardiac catheterizations Presence of leg symptoms 24.2 ⫾ 4.5 5 (3–7) 4 (2–6) 0.03 0 (0–0) ⬍0.0001 85 (54%) 16 (40%) 0.13 1 (4%) ⬍0.0001 Data are expressed as mean ⫾ SD, median (interquartile range), or n (%). *Age at surgical repair indicates age at Fontan completion surgery and complete repair of tetralogy of Fallot (TOF). interquartile ranges depending on normality. Categorical data are reported as percents. Patient characteristics were compared between the Fontan group and the control and comparison groups using t tests, chi-square tests, and Fisher exact tests, as appropriate. CVI was defined as CEAP grade ⱖ1 and severe CVI as CEAP grade ⱖ4. The prevalence of CVI in the Fontan group was compared with the control and comparison groups using chi-square or Fisher exact tests. The rates of severe venous insufficiency were similarly compared. Within the group of Fontan patients, univariate logistic regression was used to identify demographic and clinical risk factors of the binary outcome of severe CVI. Factors with univariate p values ⬍0.05 were considered for inclusion in a multivariate logistic regression model. To prevent overfitting, only models with at most 3 predictors were considered. The final model was the one that maximized the likelihood score statistic. Interobserver agreement among the subset of patients who were re-reviewed was quantified with the weighted kappa coefficient. For all tests, statistical significance was defined by a 2-sided p value ⬍0.05. Statistical analyses were performed using SAS version 9.2 for Windows (SAS Institute Inc., Cary, North Carolina). cent of the repaired TOF group reported lower extremity symptoms, compared with only 1 patient (4%) in the healthy control group. Table 3 summarizes the anatomical and clinical characteristics in the Fontan subjects. The 159 Fontan subjects were 30.6 ⫾ 9.1 years of age at enrollment. The mean body mass index of the cohort was 24.2 ⫾ 4.5 kg/m2, and the mean resting oxygen saturation was 92.6 ⫾ 4.3%. The distribution of ventricular morphologic subgroups was as follows: left ventricle, 71%; right ventricle, 26%; and mixed, 3%. Eighty-three percent of subjects had undergone some Anatomical Fontan Subjects and Clinical Characteristics Fontan Subjects Table 3 Anatomical and Clinical Characteristics Characteristic Fontan Patients (n ⴝ 159) Cardiac diagnosis Tricuspid atresia 54 (34%) Double-inlet LV 41 (26%) Heterotaxy syndrome 21 (13%) Hypoplastic left heart syndrome 4 (2%) Unbalanced atrioventricular canal 4 (2.5%) Pulmonary atresia, intact ventricular septum Other single ventricle 3 (1.9%) 32 (20.6%) Type of Fontan procedure Results Atriopulmonary 70 (44%) Lateral tunnel 57 (36%) Overall cohort. A total of 159 adults with Fontan physiology were enrolled at 7 U.S. adult congenital centers. The control and comparison groups were enrolled at the core center and consisted of 25 healthy subjects without heart disease and 40 subjects with repaired TOF. The baseline characteristics of this cohort are summarized in Table 2. The mean age (32.1 ⫾ 7.6 years), sex distribution (40% male), and body mass index (23.1 ⫾ 2.5 kg/m2) were statistically comparable between the healthy control group (p ⫽ NS) and the Fontan cohort. No subjects in the healthy control group had undergone prior cardiac catheterization. The repaired TOF group had a similar sex distribution (40% male) and mean body mass index (25.7 ⫾ 5.9 kg/m2) (p ⫽ NS), but they were slightly older (mean age 35.6 ⫾ 10.7 years; p ⫽ 0.003) than the Fontan group. The repaired TOF group had a median of 4 (interquartile range 2 to 6) cardiac catheterizations with prior groin access. Forty per- Atrioventricular 13 (8%) Extracardiac conduit Other 9 (6%) 10 (6%) Vascular history Deep venous thrombosis 10 (6.5%) Fontan pathway thrombosis 24 (15.7%) Pulmonary thrombus/embolism 10 (6.5%) Medication use Antiarrhythmic agents 54 (34.2%) ACE inhibitors/ARBs 84 (53.2%) Beta-blockers 39 (31%) Diuretic agents 71 (44.9%) Warfarin 91 (57.6%) Aspirin 70 (44.3%) Intracardiac devices Pacemaker Internal defibrillator 57 (35.8%) 2 (1.3%) ACE ⫽ angiotensin-converting enzyme; ARB ⫽ angiotensin II receptor blocker; LV ⫽ left ventricle. Valente et al. Venous Disease in Adult Fontan Patients JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 147 prevalence of severe venous insufficiency (CEAP grade ⱖ4) (Figs. 1 to 3) was significantly higher in the Fontan cohort (22%; 95% CI: 16% to 29%) versus the healthy control group (0%; 95% CI: 0% to 14%) (p ⫽ 0.005). There was no difference in the prevalence of CVI between the Fontan group (22%; 95% CI: 16% to 29%) and the subjects with repaired TOF (12.5%; 95% CI: 4% to 27%) (p ⫽ 0.27). Univariate and multivariate predictors of severe CVI in the Fontan population are summarized in Table 4. The kappa value was 0.76 for interobserver CEAP classification. Of importance, 100% concordance was observed for patients denoted as having severe disease (CEAP grade ⱖ4). Discussion Figure 1 35-Year-Old Woman, Status Post-Fontan Revision to an Extracardiac Conduit 3 Years Before Enrollment Clinical, etiological, anatomical, and pathophysiological (CEAP) grade 4 with skin pigmentation and lipodermatosclerosis. surgical palliation before Fontan completion. The mean age at the initial Fontan procedure was 11.2 ⫾ 9.3 years, and the most common types of Fontan procedures performed were atriopulmonary anastomosis and intracardiac lateral tunnel. Twenty-eight patients had undergone subsequent Fontan revision, and 31 had undergone subsequent Fontan fenestration closure. Subjects in the cohort had undergone a median of 5 (interquartile range 3 to 7) cardiac catheterizations with femoral intravenous access, and 10% had documented femoral venous occlusion. Of the 147 patients who had imaging data within 1 year of enrollment, over 40% of the cohort had at least mild systemic ventricular dysfunction, and ⬎70% had at least mild atrial ventricular valve regurgitation. On direct questioning regarding symptoms relating to systemic venous disease and health, 85 subjects (54%) reported at least 1 of the following: burning, swelling, pain, itching, or leg heaviness. Thirty-five percent of the cohort was involved in activities that required long periods of standing. Ten subjects had histories of deep venous thrombosis of the lower extremity, and 10 additional subjects had received diagnoses of pulmonary emboli in the past. Additionally, 24 subjects had histories of thrombosis in the Fontan pathway, and 9 had experienced cerebral vascular accidents after Fontan completion. Forty percent reported family histories of lower extremity venous disease. Twentythree percent had histories of tobacco abuse, 10% had diabetes, and 27% of the women had completed at least 1 pregnancy. Prevalence. The prevalence of CVI was significantly greater in the Fontan population (60%; 95% confidence interval [CI]: 52% to 68%) compared with healthy controls (32%; 95% CI: 15% to 54%) (p ⫽ 0.008). Strikingly, the This multi-institutional, prospective, cross-sectional assessment is the first to document the high prevalence of CVI in adults with congenital heart disease and Fontan physiology; strikingly, the point prevalence of severe CVI in the Fontan population examined in this study approached 25%. This appears to be more than 3 to 4 times higher than the point prevalence of severe CVI reported in the general population, although it approximates that seen in other groups (older age, deep venous thrombosis) thought to be at high risk for severe CVI (12,23). Of interest, this prevalence of severe CVI in our cohort is similar to that reported for systemic venous pathway thrombosis in a single-center study of adult Fontan outpatients (24). Several mechanisms contribute to the development and maintenance of CVI, each of which is particularly relevant to adult survivors with Fontan circulation. Pressure in lower Figure 2 41-Year-Old Man Status Post-Right Atrial Appendage to Pulmonary Artery Fontan at Age 18 Years CEAP grade 5 with healed skin ulceration. Abbreviation as in Figure 1. 148 Figure 3 Valente et al. Venous Disease in Adult Fontan Patients 43-Year-Old Man Status Post-Atriopulmonary Fontan Procedure at Age 5 Years CEAP grade 6 with extensive skin changes and area of active ulceration. Abbreviation as in Figure 1. extremity veins is determined by 2 components: hydrostatic forces related to the weight of the column of blood from the right atrium to the foot and a hydrodynamic determinant related to pressures generated by contractions of the skeletal muscles of the leg and the pressure in the capillary network (25). Concomitant with the development of CVI, cellular activation and cytokine release involving leukocyte interaction with the endothelium lead to an inflammatory and potentially destructive process involving pathways similar to those activated in chronic heart failure syndromes (26). The hydrostatic component to the elevation of systemic venous pressures may be increased in patients with Fontan palliation because of several factors. This unique circulatory model intricately ties the loading conditions of the central pump to loading conditions in the systemic veins, in contrast to the normal circulation, which has an intervening pump in the subpulmonary position. By the very definition of this physiology, right atrial pressure is greater than left atrial pressure, leading to a reversal of the typical trend seen in normal subjects, as well as to increased systemic venous JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 pressure. Any additional resistance to flow, from the peripheral systemic veins through the right atrium and lungs, leads to further elevation of hydrostatic pressure in the systemic veins. With increasing heart rates, survivors with Fontan circulation demonstrate limited inotropic response and worsening of diastolic filling of the systemic arterial pump, leading to elevation of left atrial pressure and, because of direct coupling across the pulmonary vascular bed, elevation in central venous pressure (4). Reduction of normal respiratory changes in transhepatic systemic venous flow and an increase in transhepatic venous resistance, observed in adult survivors with Fontan physiology, may further exacerbate distal systemic venous pressures (27). Taken together, the elevation of distal and more proximal systemic venous pressures leads to distention and engorgement of the veins, increased pressure on local tissue, and ultimate induction of inflammation in the vascular wall, venous valves (with development of valve remodeling, loss of function, and reflux) (28 –30) and local tissues. Additional hydrodynamic components leading to development of CVI in Fontan circulations have been less examined. Inai et al. (8) recently demonstrated altered skeletal muscle hemodynamic conditions in patients with Fontan palliation, although calf pump function was not specifically explored. Systemic venous valve dysfunction, caused by high systemic venous pressures and inflammatory microcirculation changes outlined previously, likely contribute to increased risk for venous thromboembolism and, as well, are likely worsened by the heightened occurrence of such venous thromboembolism in patients with Fontan palliation (due in part to circulating prothrombotic factors [31]) throughout the Fontan pathway (2). The recognition and cataloguing of CVI in the population of adult congenital heart disease survivors is a new finding and was aided by the collaborative efforts of a multi-institutional research network. Multiple patient factors were identified as predictors of severe CVI, including age, male sex, and prior occurrence of deep venous thrombosis. Adult survivors with Fontan palliation frequently underwent catheterization using venous access at a much younger age and smaller vessel size than the population of other adults with CVI and associated coronary atherosclerosis (typically using arterial access), potentially leading to greater degrees of venous obstruction and inflammation and ultimately to increased occurrence of CVI. Interestingly, cardiac findings traditionally considered to be risk factors for adverse outcomes in adults with Fontan physiology did not correlate with increased risk for CVI. These include morphologic systemic ventricle type and surgical type of Fontan procedure (atriopulmonary anastomosis vs. lateral tunnel procedure). Additionally, prescribed patient use of anticoagulant agents did not predict the severity of CVI. One concerning, although important, finding in this cohort is the lack of a significant difference between the prevalence of CVI in the adult Fontan population compared Valente et al. Venous Disease in Adult Fontan Patients JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 149 Univariate Multivariate Predictors for Severe CVI Fontan Patients Table 4 and Univariate and Multivariate Predictors forinSevere CVI in Fontan Patients Univariate Multivariate Odds Ratio (95% CI) p Value Male sex 2.42 (1.12–5.25) 0.02 Age ⱖ31 yrs 6.54 (2.80–15.27) ⬍0.0001 Single right ventricle 0.41 (0.15–1.13) 0.07 Atriopulmonary Fontan 3.64 (1.36–9.79) 0.03 Atrioventricular Fontan 5.31 (1.31–21.57) Extracardiac Fontan 2.43 (0.41–14.47) Other Fontan type 0.95 (0.10–8.80) Variable Catheterizations ⱖ5 Odds Ratio (95% CI) p Value 5.68 (2.30–14.02) 0.0002 5.40 (1.92–15.18) 0.001 10.12 (4.30–23.82) ⬍0.0001 11.13 (4.25–29.11) ⬍0.0001 Diuretic agent use 5.01 (2.16–11.62) ⬍0.0001 Antiarrhythmic agent use 2.99 (1.38–6.46) 0.005 Heart failure diagnosis 2.46 (1.12–5.40) 0.03 History of DVT 6.15 (1.63–23.24) 0.007 5.80 (1.29–26.01) 0.02 History of venous surgery 3.78 (0.73–19.63) 0.12 Body mass index 1.11 (1.12–1.20) 0.01 Lower extremity itching CI ⫽ confidence interval; CVI ⫽ chronic venous insufficiency; DVT ⫽ deep venous thrombosis. with the comparison group of adults with repaired TOF. The high prevalence of CVI in this slightly older latter group suggests that these patients, as well, may have adverse peripheral hemodynamic status, which remains to be explored. Patients with repaired TOF are a limited comparison population given the combination of potential confounding effects such as elevated systemic venous pressures and subpulmonary ventricular dysfunction that may exist in this group. Adults with Fontan physiology are among the most challenging groups followed in adult congenital clinics, with high morbidity and mortality. Thromboembolism, exercise incapacity, and heart failure appear common and increase both in incidence and severity over time. The association between peripheral hemodynamic status, CVI, and endothelial dysfunction may prove to both depend on, as well as contribute to, the long-term consequences and decreased functional capacity (32) associated with Fontan physiology. As was shown by assessment of the comparison group with TOF, examination of this unique patient population underscores the importance of examining the peripheral circulation in all adult patients with congenital heart disease, to determine its contribution to mechanisms of heart failure and exercise intolerance. Ultimately, recognition and determination of the mechanisms of CVI in survivors with Fontan palliation may lead to the assessment of interventions with proven benefit in the general CVI population (such as dedicated lower extremity muscle training exercises and venous compression stockings) in the Fontan population. Pharmacological therapies to decrease systemic venous pressure have not been shown to be effective in the general population, but the benefit of such therapies on the occurrence of highly morbid and mortal outcomes in the Fontan population remains unknown. Study limitations. This study had several important limitations. This cross-sectional observational study was performed at 7 major adult congenital heart centers. The study patient population may not be representative of all adults with Fontan physiology in the general community, because of a referral bias. However, it is recognized that the majority of patients with Fontan palliation remain in care at such institutions. Furthermore, this study was limited to measurements at a single physical assessment. Therefore, changes in systemic venous health and disease over time could not be assessed. However, this study lays the groundwork for the implementation of longitudinal examination of the functional relationships and potential interventions in this population. Given a possible association between CVI and mortality, survivor bias is a concern. This would tend to bias the findings toward a lower proportion of patients with CVI, however, because those who died would likely have a higher prevalence of CVI. Finally, the lack of significant difference between the prevalence of CVI in the adult Fontan population compared with the comparison group of adults with repaired TOF may reflect the age difference between the 2 groups or a lack of power in the study due to inadequate sample size. Conclusions CVI is common in adult congenital heart patients with Fontan physiology and is often severe. The occurrence of CVI in other populations of adults with congenital heart disease, as well as the contribution of abnormal peripheral hemodynamic conditions in adult survivors with Fontan palliation to comorbidities including thromboembolism and heart failure, and interventions to improve peripheral hemodynamics require further exploration. 150 Valente et al. Venous Disease in Adult Fontan Patients JACC Vol. 56, No. 2, 2010 July 6, 2010:144–50 Acknowledgments The authors thank the participants in this study, as well as the numerous cardiologists and cardiothoracic surgeons who have been involved in the care of these patients. The authors also thank Elizabeth Rodriguez-Huertas, Terra Lafranchi, NP, and Sarah Evans of the Boston Adult Congenital Heart Research Team; Handan Titiz, EdM, of the Children’s Hospital Boston Clinical Research Program; Deborah Shellenberger, PA-C, at the University of Pennsylvania; and Mary Elizabeth Schlater, NP, at the University of Washington. The authors additionally thank Joseph Kay, MD, Jennifer Ting, MD, Craig Broberg, MD, Michelle Nickolaus, NP, Carole Warnes, MD, and Karen Kuehl, MD, for their collaboration through the Alliance for Adult Research in Congenital Cardiology group. Reprint requests and correspondence: Dr. Anne Marie Valente, Boston Adult Congenital Heart Program, Department of Cardiology, Children’s Hospital Boston, 300 Longwood Avenue, Boston, Massachusetts 02115. E-mail: [email protected]. REFERENCES 1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240 – 8. 2. Khairy P, Fernandes SM, Mayer JE Jr., et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85–92. 3. Ghanayem NS, Berger S, Tweddell JS. Medical management of the failing Fontan. Pediatr Cardiol 2007;28:465–71. 4. Senzaki H, Masutani S, Ishido H, et al. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol 2006;47:2528 –35. 5. Shiraishi S, Yagihara T, Kagisaki K, et al. Impact of age at Fontan completion on postoperative hemodynamics and long-term aerobic exercise capacity in patients with dominant left ventricle. Ann Thorac Surg 2009;87:555– 61. 6. Ohuchi H, Wakisaka Y, Watanabe K, Kishiki K, Yamada O, Echigo S. Impact of central hypercapnic chemosensitivity on enhanced ventilation in patients after the Fontan operation. Int J Cardiol 2007;121: 36 – 43. 7. Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99 –107. 8. Inai K, Saita Y, Takeda S, Nakazawa M, Kimura H. Skeletal muscle hemodynamics and endothelial function in patients after Fontan operation. Am J Cardiol 2004;93:792–7. 9. Binotto MA, Maeda NY, Lopes AA. Altered endothelial function following the Fontan procedure. Cardiol Young 2008;18:70 – 4. 10. Mahle WT, Todd K, Fyfe DA. Endothelial function following the Fontan operation. Am J Cardiol 2003;91:1286 – 8. 11. Jin SM, Noh CI, Bae EJ, Choi JY, Yun YS. Impaired vascular function in patients with Fontan circulation. Int J Cardiol 2007;120:221– 6. 12. Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg 2003;37:1047–53. 13. Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. general population: Edinburgh Vein Study. J Epidemiol Community Health 1999;53:149 –53. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83–102. Moffatt CJ, Franks PJ, Doherty DC, Martin R, Blewett R, Ross F. Prevalence of leg ulceration in a London population. Q J Med 2004;97:431–7. Kistner RL. Definitive diagnosis and definitive treatment in chronic venous disease: a concept whose time has come. J Vasc Surg 1996;24: 703–10. Fowkes FG, Lee AJ, Evans CJ, Allan PL, Bradbury AW, Ruckley CV. Lifestyle risk factors for lower limb venous reflux in the general population: Edinburgh Vein Study. Int J Epidemiol 2001;30:846 –52. Eklof B, Rutherford RB, Bergan JJ, et al., American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248 –52. Porter JM, Moneta GL, International Consensus Committee on Chronic Venous Disease. Reporting standards in venous disease: an update. J Vasc Surg 1995;21:635– 45. Khairy P, Hosn JA, Broberg C, et al. Multicenter research in adult congenital heart disease. Int J Cardiol 2008;129:155–9. Franklin RC, Jacobs JP, Krogmann ON, et al. Nomenclature for congenital and paediatric cardiac disease: historical perspectives and The International Pediatric and Congenital Cardiac Code. Cardiol Young 2008;18 Suppl:70 – 80. Padberg F Jr., Cerveira JJ, Lal BK, Pappas PJ, Varma S, Hobson RW II. Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg 2003;37:79 – 85. Criqui MH, Denenberg JO, Bergan J, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego Population Study. J Vasc Surg 2007;46:331–7. Varma C, Warr MR, Hendler AL, Paul NS, Webb GD, Therrien J. Prevalence of “silent” pulmonary emboli in adults after the Fontan operation. J Am Coll Cardiol 2003;41:2252– 8. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2005;111:2398 – 409. Bergan JJ, Pascarella L, Schmid-Schönbein GW. Pathogenesis of primary chronic venous disease: insights from animal models of venous hypertension. J Vasc Surg 2008;47:183–92. Hsia TY, Khambadkone S, Deanfield JE, Taylor JF, Migliavacca F, De Leval MR. Subdiaphragmatic venous hemodynamics in the Fontan circulation. J Thorac Cardiovasc Surg 2001;121:436 – 47. Takase S, Pascarella L, Bergan JJ, Schmid-Schönbein GW. Hypertension-induced venous valve remodeling. J Vasc Surg 2004;39: 1329 –34. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg 2004;28:484 –93. Pascarella L, Schmid-Schönbein GW, Bergan J. An animal model of venous hypertension: the role of inflammation in venous valve failure. J Vasc Surg 2005;41:303–11. Odegard KC, McGowan FX Jr., Zurakowski D, et al. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg 2003;125:1260 –7. Brassard P, Bédard E, Jobin J, Rodés-Cabau J, Poirier P. Exercise capacity and impact of exercise training in patients after a Fontan procedure: a review. Can J Cardiol 2006;22:489 –95. Key Words: Fontan y venous insufficiency y adult congenital heart disease.