* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Phases of Matter and Phase Changes
100% renewable energy wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Regenerative brake wikipedia , lookup
Energy storage wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
World energy consumption wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
International Energy Agency wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Zero-energy building wikipedia , lookup
Alternative energy wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Energy harvesting wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Micro combined heat and power wikipedia , lookup
Distributed generation wikipedia , lookup
Negawatt power wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
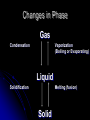
Phases of Matter, Energy and Phase Changes Phase of Matter . Depends on strength of forces of attraction between particles. Solids Definite shape and volume. Most dense phase (Exception is water!) Difficult to compress. Particles vibrate in fixed positions Crystalline lattice structure. Most attraction between particles. Note: Amorphous solids include glass, plastic, wax, and silly putty Liquids Definite volume No definite shape Hard to compress Particles slide past each other Forces of attraction between particles still high Gases No definite shape or volume Expands to fill container Lowest density Little attraction between particles “Vapor” = a gaseous state of something that is normally liquid (Ex: water vapor) Changes in Phase Gas Condensation Vaporization (Boiling or Evaporating) Liquid Solidification Melting (fusion) Solid Phase Changes Short Summary video on phases: (1 min) http://www.youtube.com/watch?v=s-KvoVzukHo&safe=active Applet: (Excellent) https://phet.colorado.edu/en/simulation/states-of-matter http://www.harcourtschool.com/activity/states_of_matter/ Let’s Skip a Phase Sublimation Directly from the solid phase to the gas phase. Happens with substances with weak intermolecular forces of attraction They separate easily! Ex: CO2(s) dry ice, Iodine CO2(s) → CO2 (g) http://www.youtube.com/watch?v=8tHOVVgGkpk Energy Energy = capacity to do work or produce heat. It can be anything that causes matter to move or change direction. Many different types of energy Ex: electrical, thermal, atomic, mechanical “Chemical” energy is the potential energy stored in the bonds between atoms Law of Conservation of Energy Energy can’t be created or destroyed, just transferred from one form to another PE vs. KE Potential Energy stored energy Energy can be stored in bonds between atoms Kinetic Energy energy of motion All atoms are moving and vibrating unless at absolute zero Energy and Changes to Matter Exothermic Change: A + B → C + D + energy Energy is released or “ex”its Endothermic Change: A + B + energy → C + D Energy is absorbed or “en”ters Energy During Phase Changes Solid Liquid or Liquid Gas Endothermic Energy is absorbed and overcomes attractive forces between particles Add heat Gas Liquid, Liquid Solid Exothermic As particles come closer together energy is released Remove heat Heat Energy Also called Thermal energy, it makes particles move more as it is added Measured in Joules or calories. http://www.youtube.com/watch?v=f1eAOygDP5s&safe=active Heat Flow or Transfer Heat energy travels from an object of higher temp. to one of lower temp. until both reach the same temp. Temperature Measure of the average kinetic energy (motion) of all the particles in a sample. Not a form of energy!!! But if you add heat energy or take it away, it causes particles to move faster or slower and thus changes the temp. Heat vs Temp. https://www.youtube.com/watch?v=yxBTE MnrZZk Heat vs. Temperature Teacup vs. Bathtub Both at 25˚C Which one contains more heat energy? Which one has the greater average KE? Temperature Scales Used in Chemistry Celsius Fixed points of scale based on the freezing point and boiling point of water 0 °C = water freezes, 100 °C = water boils Kelvin Scale based on lowest temperature possible 0 K = absolute zero https://www.youtube.com/watch?v=-G9FdNqUVBQ Temperature Scales and Conversions K = ˚C + 273 Absolute Zero Temperature at which particles have slowed down so much they no longer possess any kinetic energy. 0 Kelvin -273° Celsius Heating & Cooling Curves Graphically represents temp. changes as heat energy is added or taken away. Label This Graph Interpreting the Graph The slanted portions = temp is changing Single phase is heating up or cooling down KE is changing The flat portions = temp not changing Substance undergoing a phase change PE is changing Heating Curve for Water Label Melting Pt? Boiling Pt?