* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Gustatory and Olfactory Systems - Dr. Costanzo
Survey
Document related concepts
Transcript
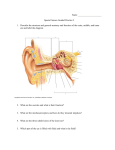
Gustatory and Olfactory Systems Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the location and morphological characteristics of the sensory organs responsible for taste and smell. 2. Describe the innervation for each of the cranial nerves that mediate: a. Taste sensations b. Smell sensations 3. Describe the spatial distribution of taste sensations across the different regions of the tongue. 4. Describe a mechanism by which olfactory stimuli might be encoded by the nervous system. 5. Identify clinical disorders that could result in impairment of gustatory or olfactory function. 6. Compare and contrast the features of the gustatory and olfactory systems with those of other sensory systems. I. GUSTATION (THE CHEMICAL SENSE OF TASTE) A. TASTE BUDS The receptor cells for taste stimuli are located in structures called TASTE BUDS. These taste buds are found primarily distributed over the surface of the tongue in bulges or projections called PAPILLAE. Some taste buds are also found on the soft palate, epiglottis and lower oral pharynx. Figure 1: Structure of the taste bud (from Costanzo, 2006) Individual taste buds are made up of specialized epithelial cells (approximately 40-50 cells per bud) which form a barrel-shaped structure. At the surface of each taste bud is a small fluid-filled opening in the epithelium called the taste pore. It is through this pore that chemical stimuli reach the taste receptor cells. There are three cell types within the taste buds: 1. BASAL CELLS 2. SUPPORTING CELLS 3. SENSORY CELLS (also called TASTE RECEPTOR CELLS) The BASAL CELLS are undifferentiated, stem cells located near the base of the taste bud. In the normal adult, basal cells undergo a process of continuous regeneration. Every 10 days a new cell is produced which migrates toward the center of the taste bud where it differentiates into either a sensory or supporting cell. Basal cells replace old cells which die off and presumably pass through the taste pore to the surface of the tongue. This unique capacity to replace sensory receptor cells appears to be limited to the chemical senses. As you will see later, a similar process takes place in the olfactory epithelium. The SUPPORTING CELLS are found distributed among the receptor cells within the taste bud. They are similar in appearance to receptor cells but do not respond to taste stimuli. The function of the supporting cells is not known. The SENSORY CELLS or TASTE RECEPTOR CELLS are covered with numerous microvilli. These microvilli are found at the apical surface of the cell and project into the taste pore. Because of their location and large surface area, microvilli provide ideal sites for the transduction of chemical stimuli into electrical currents (receptor potentials). It should be emphasized that taste receptor cells are not neurons. They do not have axons and they do not transmit action potentials to the central nervous system. The cell bodies of the first order taste neurons are located outside the gustatory epithelium, and only their nerve fibers enter the taste bud where they make synaptic connections with the sensory receptor cells. A single taste nerve fiber may innervate several taste buds, and within a given taste bud may innervate several taste receptor cells. The integrity of cells within a given taste bud depends very much upon the innervation of that bud by the taste fibers. If these nerve fibers are cut and allowed to degenerate, cells within the taste bud will also degenerate and the taste bud will soon disappear. If, however, the nerve is allowed to regenerate and grow back into the epithelium, a new taste bud will develop in the region where the nerve ending terminates. B. PAPILLAE Taste buds located on the tongue are always associated with specialized papillae. There are four types of papillae in man. These are the FUNGIFORM, FILIFORM, FOLIATE and CIRCUMVALLATE (VALLATE) papillae. Figure 2: Types of papillae found on the tongue FUNGIFORM papillae are scattered across the dorsal surface of the tongue. They are most numerous near the anterior tip. These mushroomshaped bulges contain 3-5 taste buds which are located on the dorsal surface. Fungiform papillae can be easily identified as red spots on the surface on the tongue. This is due to the rich blood supply which is located just beneath the taste buds. The FOLIATE papillae are located on the lateral border of the tongue. In this case, the taste buds are in folds on the sides of the papillae. CIRCUMVALLATE papillae are the largest of all papillae. They are few in number (only 10-12 in man) and are arranged in rows near the base of the tongue. Each circumvallate papillae is surrounded by a circular furrow or trench and numerous taste buds are located in the sides of these furrows. In fact, nearly half of the taste buds in the tongue are located on the circumvallate papillae (it has been estimated that there are as many as 250 buds per circumvallate papillae). The FILIFORM papillae make up the fourth type. They are leaf-shaped, 2-3 mm long and do not contain taste buds. In spite of the absence of taste buds, the filiform are the most abundant type of papillae found on the surface on the tongue. C. GUSTATORY NERVES The cranial nerves involved in taste pathways are: 1. FACIAL (VII) 2. GLOSSOPHARYNGEAL (IX) 3. VAGUS (X) The FACIAL NERVE (VII) subserves taste over the anterior two-thirds on the tongue. Fibers from the anterior tongue run through the lingual nerve, chorda tympani and facial nerve to the geniculate ganglion. The cell bodies of these taste fibers are in the GENICULATE GANGLION. The GLOSSOPHARYNGEAL NERVE (IX) innervates the posterior third of the tongue and its fibers have cell bodies originating in the PETROSAL GANGLION. Finally, taste fibers in the VAGUS NERVE (X) innervate the epiglottis and lower pharynx. Cell bodies of the vagus nerve are located in the NODOSE GANGLION. Figure 3: Nerves innervating the tongue The TRIGEMINAL NERVE (V) also innervates the tongue but is not considered to be involved in gustation. This nerve travels together with the chorda tympani fibers in the lingual nerve to innervate the anterior twothirds of the tongue. The cell bodies are located in the GASSERIAN GANGLION and are involved in touch, temperature and pain. D. CENTRAL TASTE PATHWAYS Figure 4: Central taste pathways The nerve fibers mediating taste (VII, IX and X) enter the medulla and ascend together in a bundle called the SOLITARY FASCICULUS or TRACT. These nerves terminate on the second order taste cells located in the rostral portion of the SOLITARY NUCLEUS. Taste cells in the SOLITARY NUCLEUS project primarily ipsilaterally to the VENTRAL POSTEROMEDIAL NUCLEUS (VPM) of the thalamus. Fibers projecting to the thalamus (VPM) probably travel in the central tegmental tract and not the medial lemniscus. From the thalamus, there are projections to cells in a region of the neocortex known as the "CORTICAL TASTE AREA". This region includes the LATERAL PORTION of the POSTCENTRAL GYRUS (the face-tongue area of SI) and the OPERCULAR INSULAR CORTEX. Gustatory information also reaches areas of the BASAL FOREBRAIN including the hypothalamus, amygdaloid complex and the bed nucleus of the stria terminalis. It has been suggested that projections to the basal forebrain may play a role in the regulation and control of food intake and drinking behavior. E. BASIC TASTE SENSATIONS (TASTE QUALITIES) The four basic taste sensations are usually identified as SALTY, SWEET, SOUR AND BITTER. A fifth taste called UMAMI is used to describe the flavor-enhancing taste of monosodium glutamate (MSG). Several transduction mechanisms are involved in mediating taste sensations. Salt and sour sensations are mediated by a direct interaction of chemicals with ion channels. For example the taste of table salt (NaCl) is mediated by an Amiloride sensitive sodium channel located on taste bud receptor cells. Sour (H+) taste is mediated by inhibition of a K+ voltage sensitive channel. Sweet and bitter sensations involve second messenger systems that lead to depolarization of the receptor cells. Depolarization of the taste receptor cells cause release of neurotransmitters and activation of the taste nerves. Figure 5: Taste transduction mechanisms (from Costanzo, 2006) F. NEURAL CODING Recordings from individual single taste nerve fibers has led to the across fiber pattern code for taste. These recordings show that no two taste fibers have identical response characteristics. Each fiber responds to all four taste qualities and there are differences in the magnitude of response to each of the different stimuli. In the across fiber pattern code, a single fiber alone does not encode stimulus quality, rather the response pattern across many fibers at the same time is used to discriminate a particular stimulus. G. TASTE DISORDERS Taste disorders are not considered life threatening, however patients with a taste loss often develop problems related to diet and nutritional status, and there is an increased risk of accidental food poisoning or ingestion of toxic substances. Disorders of taste may occur after: damage to taste nerves, lesions in the cortical taste centers (head injury, stroke, and brain tumors), drug treatment, radiation of the head and neck, diseases of the oral cavity and normal aging. 1. Quantitative a. HYPOGEUSIA - a decrease in taste sensitivity b. AGEUSIA - absence of the sense of taste c. HYPERGEUSIA - an increase in taste sensitivity 2. Qualitative a. DYSGEUSIA - impairment or distortion of taste 1. CACOGEUSIA - a bad or foul sense of taste 2. PARAGEUSIA - a taste sensation in the absence of the appropriate stimuli II. OLFACTION (THE SENSE OF SMELL) A. NASAL CAVITY Odor stimuli, or smells, reach the olfactory receptors by way of the nasal cavity. During normal breathing, air enters the external nares (nostrils), passes across the nasal cavity and exits into the nasopharynx. Figure 6: The human nasal cavity Within the nasal cavity are a series of structures called the TURBINATES or CONCHAE (SUPERIOR, MIDDLE AND INFERIOR). These structures act as baffles or deflectors, causing airflow to become turbulent or nonlinear in nature. The result is that some of the air stream, is diverted to the upper regions where the olfactory receptor cells are located. The OLFACTORY EPITHELIUM lines the entire SUPERIOR TURBINATE, part of the MIDDLE TURBINATE and the upper part of the NASAL SEPTUM. This region is yellowish brown in color and occupies an area of about 2-5 cm2. Some 100 million (108) olfactory receptor cells are concentrated in this region. The rest of the nasal cavity contains RESPIRATORY EPITHELIUM which in contrast to the olfactory epithelium is reddish pink in color. This region contains ciliated epithelial cells which beat rhythmically and aid in the movement of mucous across the surface of the epithelium. Figure 7 Netter Presenter III.84 B. OLFACTORY EPITHELIUM The olfactory epithelium consists of three basic cell types: 1. BASAL CELLS 2. SUPPORTING CELLS 3. OLFACTORY RECEPTOR CELLS Figure 8: The olfactory epithelium BASAL CELLS, located at the base of the epithelium, are undifferentiated stem cells which give rise to olfactory receptor cells. These stem cells undergo mitosis producing a continuous turnover and a supply of new supporting and receptor cells. This process takes place throughout the adult life. Continuous neurogenesis of olfactory receptor cells is similar to that which occurs in the gustatory epithelium. However, there are few important differences. First, the time it takes a basal cell in the olfactory epithelium to develop and emerge as a mature receptor cell (25-30 days) is somewhat longer than in the gustatory epithelium. Secondly, unlike taste receptor cells, olfactory receptor cells are true nerve cells (neurons). They have a dendritic rod, an axon, and conduct nerve impulses (action potentials) into the central nervous system. This is quite remarkable since these cells are neurons, and neurons in the adult mammalian nervous system do not replace themselves. The SUPPORTING CELLS (sometimes called SUSTENTACULARCELLS) are COLUMNAR EPITHELIAL CELLS. They are located at the border of the epithelium and are distributed homogeneously among receptor cells. They have numerous microvilli which extend into the olfactory mucosa and contain secretory granules which are located near the surface and empty their contents into the mucosal layer. It may be that this secretory process is an important function of the supporting cells. The RECEPTOR CELLS are flask-shaped cells and have relatively large cell bodies (5-8 microns) and a thin dendritic rod of about 1-2 microns wide. At the mucosal surface, this rod forms a swelling called the olfactory vesicle or knob. The olfactory vesicle contains a number of cilia (10-15) which extend out into the mucous layer for distances up to 100 microns. These cilia have a typical 9 plus 2 pattern of filaments, and because of their close proximity to the air-mucosal surface, they are thought to be the site for transduction of odor molecules. The basal end of the receptor cells give rise to a thin unmyelinated fiber which passes out of the epithelium and travels centrally to the olfactory bulb. These olfactory nerve fibers are unique for two reasons. First, they are among the smallest fibers in the nervous system ranging in diameter from 0.1-0.3 microns and as expected they have extremely slow conduction velocities of 0.2-0.3 M/sec (very slow). The second unique property of the olfactory nerves is that within a single Schwann cell invagination, these unmyelinated fibers run together in bundles and apparently are not insulated from one another. It should be pointed out that in addition to innervation by the olfactory nerve, the olfactory epithelium is also innervated by branches of the TRIGEMINAL (V) NERVE. They are the principle detectors for noxious and caustic chemicals such as ammonia, formaldehyde and other stinging and sometimes painful odors. A basic distinction should be made between the detection of noxious stimuli by the V nerve and "olfaction" which is the discrimination and detection of odor stimuli and is by definition mediated by the olfactory nerves (1st cranial nerves). This distinction has clinical importance. For example in head injury the delicate olfactory nerves that pass through the cribriform plate of the ethmoid bone can be completely severed making the olfactory system incapable of function. However, if tested with inappropriate stimuli (i.e., alcohol, smelling salts of ammonia, vicks), patients will report that they can still smell. This phenomenon is the result of stimulating the trigeminal nerve endings within the nasal cavity and is not an olfactory sensation. Evaluation of the olfactory nerves (1st cranial nerves) requires the use of appropriate odor stimuli. Mild odors such as chocolate, peanut butter, aroma of a cup of coffee or the scent of a mild perfume are good examples of appropriate stimuli. C. OLFACTORY BULB Olfactory nerve fibers from the nasal epithelium, pass through the cribriform plate and project directly into the olfactory bulb. The olfactory bulb is a highly organized structure consisting of several distinct layers. They are: the glomerular layer, the outer plexiform layer, mitral cell body layer, inner plexiform layer and granule cell layer. The most important cell type is the MITRAL CELL. Mitral cells have apical dendrites that receive direct synaptic input from the olfactory nerve fibers. Their axons join together to form the OLFACTORY TRACT, which is the principle output of the bulb. The processing of olfactory information that takes place within the olfactory bulb is a consequence of the interconnections between different cells types. Figure 9. Netter Presenter, 2004, III.85 In the outermost layer of the bulb, the first order olfactory nerve fibers form clusters of synaptic connections with the dendrites of the second order mitral cells. These clusters are called GLOMERULI. There are approximately 50,000,000 olfactory nerve fibers that project to 45,000 mitral cells in each olfactory bulb. This means that there is a convergence of approximately 1,000:1 onto the second order mitral cells. The cell bodies of mitral cells are arranged in a thin layer (mitral cell body layer). They have a single apical dendrite, and several lateral dendrites which enter the external plexiform layer. Here they interact with the dendrites of other cell types such as the GRANULE CELLS. In the outermost glomerular layer there are cells which make connections between glomeruli. These are the PERIGLOMERULAR (PG) CELLS which are inhibitory interneurons. They provide for lateral inhibition of neighboring glomeruli. The deeper regions of the bulb (granule cell layer) contain short axon cells and the more significant granule cells. Granule cells consist of a cell body and a dendritic process with spines. They do not have axons. Most of the granule cell dendritic fields are located in the external plexiform layer where they interact with mitral cells by means of a specialized synapse called the dendrodendritic or reciprocal synapse. Under the electron microscope one can see two areas where Synaptic vesicles are in close opposition to the membrane. Vesicles on the granule cell side are more irregular and oval shaped indicating an inhibitory synapse from granule to mitral cell dendrites. Vesicles on the mitral cell side are more regular and spherical shaped indicating an excitatory synapse from mitral to granule cell. Thus, mitral cells excite; granule cell dendrites and granule cells inhibit mitral cell dendrites. Dendrodendritic synapses have been observed both in the glomerular layer (between mitral and periglomerular cells) and in the external plexiform layer (between mitral and granule cells). In each case, these synapses are excitatory in one direction and inhibitory in the opposite direction. Dendrodendritic connections in the olfactory bulb appear to serve two functions: SELF-INHIBITION and LATERAL INHIBITION. Selfinhibition limits the amount of excitability within a single mitral cell after the initial onset of the stimulus. This type of inhibition could explain why cells in the olfactory system adapt so rapidly to continuous odor stimuli. Lateral inhibition provides a means for inhibiting neighboring mitral cells via inhibitory periglomerular cells in the glomerular layer or via inhibitory granule cells in the external plexiform layer. The information coming through a particular mitral cell would be sharpened perhaps by eliminating background activity in neighboring mitral cells. If different mitral cells encoded different odors, then lateral inhibition could play an important role in improving the discrimination between odors. D. CENTRAL OLFACTORY PATHWAYS Figure 10: Central olfactory pathways The second order (mitral) cells in the olfactory bulb project to central regions of the brain via the olfactory tract. A medial division of this tract (the medial olfactory stria) sends connections to the ANTERIOR OLFACTORY NUCLEUS and the SEPTAL AREA (subcallosal and paraolfactory areas). There are also projections to the CONTRALATERAL OLFACTORY BULB by way of the ANTERIOR COMMISSURE. Fibers in the lateral division (lateral olfactory stria) project directly to the PRIMARY OLFACTORY CORTEX (Prepyriform cortex), PERIAMYGDALOID CORTEX (ENTORHINAL CORTEX) and the PREPYRIFORM CORTEX. In man there is intermediate branch of the olfactory tract (intermediate stria) that penetrates the anterior perforated substance of the basal forebrain to terminate in its nuclei. Like in other sensory systems, there are projections to the NEOCORTEX (orbital frontal cortex) via a thalamic relay (DORSAL MEDIAL THALAMUS, DM). Olfactory information reaches both the hypothalamus (feeding and reproduction) and the limbic system (emotion and sexual drive). E. NEURAL CODING The basic transduction mechanism for olfactory receptors involves a Gprotein second messenger system. Individual olfactory receptor cells respond to a wide range of different chemicals (are broadly tuned). This suggests that a single receptor cell alone may not provide sufficient information to encode odors. However, if one looks at the response profile across many receptors at the same time (across fiber pattern code), there appears to be different patterns of response for different odors. Thus each odor stimulus could produce a unique pattern of activity across the population of receptors and that pattern could encode that particular stimulus. This is known as the ACROSS FIBER PATTERN THEORY of olfaction. Figure 11: Coding of olfactory stimuli F. OLFACTION DISORDERS It has been estimated that between 1.5 and 2 million Americans experience some type of olfactory dysfunction. Certain pathological conditions result in anosmia (absence of smell sensation), hyposmia (decreased sensation) and dysosmia (distortion of smell sensation). These include head injury, trauma, upper respiratory infections, tumors of the anterior fossa, exposure to toxic chemicals, and diseases and anomalies of the nasal cavity. In addition, anosmia is symptomatic of certain endocrine dysfunctions such as Kallman's syndrome (hypogonadism). Detecting smell deficits can help to make a positive diagnosis. Olfactory disorders, while not typically life threatening, create significant disabilities that are frequently overlooked. For example, smell disorders can result in loss of appetite, weight loss, and in some cases malnutrition. Persons with smell problems are also unable to detect life threatening situations such as gas leaks, fires, and toxic substances. 1. QUANTITATIVE a. HYPOSMIA - a decrease in smell sensitivity b. ANOSMIA - absence of sense of smell c. HYPEROSMIA - an increase in smell sensitivity 2. QUALITATIVE a. DYSOSMIA - impairment or distortion of the sense of smell 1. CACOSMIA - a bad or foul sense of smell 2. PAROSMIA - a smell sensation in the absence of the appropriate odor stimulus REFERENCE Costanzo, L.S., Physiology, 3rd Edition, Saunders Elseveir, 2006, pp. 92-96. A Self-Assessment is available for this lecture. * Netter Presenter Image Copyright 2004 Icon Learning Systems. All rights reserved.