* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fluid Properties - The GATE Academy
Coandă effect wikipedia , lookup
Magnetorotational instability wikipedia , lookup
Flow measurement wikipedia , lookup
Hemodynamics wikipedia , lookup
Speed of sound wikipedia , lookup
Airy wave theory wikipedia , lookup
Lattice Boltzmann methods wikipedia , lookup
Computational fluid dynamics wikipedia , lookup
Accretion disk wikipedia , lookup
Hemorheology wikipedia , lookup
Magnetohydrodynamics wikipedia , lookup
Hydraulic machinery wikipedia , lookup
Sir George Stokes, 1st Baronet wikipedia , lookup
Aerodynamics wikipedia , lookup
Navier–Stokes equations wikipedia , lookup
Reynolds number wikipedia , lookup
Bernoulli's principle wikipedia , lookup
Fluid thread breakup wikipedia , lookup
Derivation of the Navier–Stokes equations wikipedia , lookup
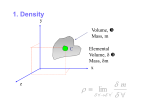
Fluid Properties Introduction A fluid is a substance which is capable of flowing or deforming under the action of shear force (however the small force may be). Examples: liquids, gases and vapors. In case of solids, deformation remains always constant with respect to time whereas in fluids deformation changes with time and hence in fluid rate of deformation is more important than deformation. The difference that exists between the solids and the fluids is in their relative abilities to resist the external force. A solid can resist tensile, compressive and shear forces up to a certain limit. A fluid has no tensile strength or very little of it, and it can resist the compressive forces only when it is kept in a container. When subjected to shear force, a fluid will deform continuously as long as this force is applied. Note: Solid can resist tangential force under static condition and fluid can resist tangential force only under dynamic condition because shearing stresses between the adjacent layers of fluid, which oppose movement of one layer of fluid over the other layer, would not exist in fluid until it comes in motion. Fluidity The tendency of continuous deformation is called fluidity and act of deformation is called flow. Reciprocal of fluidity is called viscosity. Density / Mass density / Specific mass Mass density of fluid is the mass which it possesses per unit volume at a specified temperature and pressure. It is dented by symbol ‘ρ’. Mass m Density (ρ) = volume = V Its SI unit is kg/m3. The dimension of mass density is ML-3. Mass density is proportional to the number of molecules in a unit volume of the fluid. As the temperature rises, the molecular activity and the spacing between molecules increase and hence fewer molecules exist in a given volume. Therefore, mass density decreases with increasing temperature. Also, by application of pressure more number of molecules can be forced in to a given volume thereby increasing mass density of fluid with increasing pressure. Note: 1. Maximum density of water is at 4°C and its value is 1000 kg/m3. 2. Density of air at 20°C is 1.2 kg/m3. Specific Volume It is defined as the volume of the fluid per unit mass. Specific volume is reciprocal to mass density. It is denoted by symbol ‘υ’. Its SI unit is m3/kg. Specific weight / weight density Specific weight of the fluid is defined as the weight it possesses per unit volume at a specified pressure and temperature. It is denoted by symbol ‘ω’. Weight mg ω = volume = V = ρg Its SI unit is N/m3. The dimension of weight density is ML-2T-2. Specific weight depends on temperature, pressure and location. As it varies from place to place it is not an absolute quantity. Note: 1. Weight density of water = 1000 * 9.81 = 9810 N/m3 2. For gases, the value of specific weight varies greatly with changes in pressure and temperature as compared to liquids because of the differences in the molecular structure of a gas and a liquid. In gas, the volume changes significantly on application of pressure and temperature variations. Specific Gravity / Relative density It is defined as the ratio of density (or specific weight) of any fluid to the density (or specific weight) of standard fluid. It is denoted by ‘S’. As it is a ratio of same quantity, it is dimensionless. S=ω ωfluid standard fluid =ρ ρfluid standard fluid Specific gravity shows whether a given fluid is heavier/lighter than the standard fluid. For Liquids, the standard fluid is pure water at 4°C. For gases, the standard fluid is either hydrogen or air at some specified temperature and pressure. Specific gravity of water, Swater = 1 Specific gravity of mercury, Smercury = 13.6 Viscosity Viscosity is defined as the property of the fluid by virtue of which it offers resistance to the movement of one layer of fluid over an adjacent layer of fluid. Though, viscosity is a fluid property but the effect of this property is understood only when the fluid is in motion. Viscosity in a fluid is due to 1. Cohesive forces between fluid molecules 2. Molecular momentum exchange Consider two plates placed a small distance y apart, the space between them being filled with fluid. The lower plate is assumed to be at rest, while the upper one is moved parallel to it with a velocity u by the application of a shear force F. du.dt tan dθ = dy If tan dθ is small, du.dt dθ = dy dθ du dt = dy Eqn (1) Where dθ = angular deformation or shear deformation dθ dt = rate of angular deformation or rate of shear deformation du dy = velocity gradient F Also as τ = A , hence for a given plate area if shear force F is large, τ is large. If τ is large dθ dt is large. τ dθ dt dθ τ = µ dt τ or µ = dθ dt Eqn (2) dθ We know, τ = µ dt dθ du dt = dy Hence, du τ = µ dy Eqn (3) Here ‘µ’ represents internal resistance to the flow and this is known as coefficient of viscosity or absolute viscosity or dynamic viscosity. Its SI unit is N-s/m2. The dimension of dynamic viscosity is ML-1T-1. C.G.S unit of viscosity is dyne-sec /cm2 or poise. 1 Poise = 0.1 N-s/m2 Note: 1. The viscosity of water at 20°C is 10-3 N-s/m2 and that of air at 20°C is 2 * 10-5 N-s/m2. Therefore, ( µw )at 20°C = 50 µair Kinematic Viscosity: The ratio of dynamic viscosity of fluid to the density of fluid is commonly known as kinematic viscosity. Kinematic viscosity indicates the momentum transfer to the frictional forces in the fluid flow. It is denoted by ‘ . µ =ρ Its SI unit is m2/sec. In C.G.S, the unit of kinematic viscosity is stokes. 1 stokes = 10-4 m2/sec Kinematic viscosity of air, air Kinematic viscosity of water, = µair = 1.66 * 10-5 m2/sec ρair water = µwater = 10-6 m2/sec ρwater Variation of Viscosity with temperature In case of liquids, the intermolecular distance is small and hence cohesive forces (forces of attraction between same nature molecules) are large and dominate over molecular momentum exchange. With increase in temperature, the cohesive forces decrease and hence the resistance to the flow also decreases. Therefore in case of liquids with increase in temperature, viscosity of the liquids decreases. In case of gases, intermolecular distance is very large and hence cohesive forces are negligible. Therefore in gases, molecular momentum exchange dominates over cohesive forces. With increase in temperature, molecular disturbance increases which in turn increases resistance to flow. Therefore, viscosity of gases increases with increase in temperature. Compressibility The property by virtue of which fluid undergoes change in its volume under the action of external pressure is known as compressibility (β). As long as pressure increases, the compressibility will goes on decreases. Compressibility is measured by Bulk modulus (K) of fluid. It is reciprocal of Bulk Modulus. 1 β=K Bulk Modulus It is the measure of elasticity of the fluid. Bulk Modulus for liquids are very high so liquids are treated as incompressible fluid. For water, K= 2.06 * 109 N/m2 For air, K = 1.03 * 105 N/m2 So the air is 20000 times compressible than water. Direct stress dP Bulk Modulus = Volumetric strain = -dV V Eqn (5) Where V is volume and ρ is density. Case- 1 Bulk Modulus under Isochoric condition For isochoric or constant volume condition dV = 0 dP therefore, K = -dV = ∞ V Case-2 Bulk Modulus under constant pressure or isobaric condition For isobaric condition, P= Constant dP = 0 dP therefore, K = -dV = 0 V Case-3 Bulk Modulus of ideal gas under isothermal condition or Isothermal Bulk Modulus (KT) For an ideal gas, PV= mRT m P= V R T P= ρRT Eqn (6) Also m= ρ * V ln ρ + ln V = ln m (m= constant) dρ dV ρ + V =0 -dV dρ V = ρ Hence from Eqn (5) dP K= dρ ρ Eqn (7) For isothermal process, T = Constant dP P = RT = dρ ρ dP P= dρ ρ From Eqn (7) KT = P Similarly, for adiabatic condition (K)adiabatic = γ P Where γ is adiabatic index and pressure is absolute. Classification of fluids Fluids Newtonian Fluids Non-Newtonian Fluids Time independent Dilatent Pseudoplastic Bingham plastic Time dependent Thixotropic Rheopectic Newton’s law of viscosity A fluid is said to be Newtonian fluid if shear stress is directly proportional to rate of angular deformation or rate of shear strain or velocity. τ dθ dt or τ du dy du For a Newtonian fluid, τ = µ dy du All fluids which obey Newton’s law of viscosity or the fluids which obey τ = µ dy are known as Newtonian fluids. For a Newtonian fluid, viscosity µ is constant. Example of Newtonian Fluid: Air, water, Petrol, Diesel, Kerosene, oils, Mercury Non- Newtonian Fluids Fluids which do not obey Newton’s law of viscosity are known as Non-Newtonian fluids. The study of Non-Newtonian fluids is called as Rheology. du The general relationship between shear stress (τ) and velocity gradient dy is given by du τ = τs + C dy n Eqn (4) where, τs = threshold stress n = fluid behavior index Case-1 Dilatant fluids (shear thickening fluids) From equation (4) du τ = τs + C dy n du τ = τs + C dy n 1 du dy For dilatant fluids, τs = 0 du τ = C dy n 1 du dy du dy τ = µapp du where, µapp (apparent viscosity) = k dy n 1 and n - 1 0 or n 1 For dilatant fluids, µapp increases with rate of deformation and hence dilatant fluids are also called as shear thickening fluids. Examples of dilatant fluid: Rice starch and Sugar in water Case-2 Pseudo plastic fluid (Shear thinning fluids) From equation (4) du τ = τs + C dy n du τ = τs + C dy n 1 du dy For pseudo plastic fluids, τs = 0 and n du τ = C dy n 1 1 du dy du dy τ = µapp For pseudo plastic fluids, the apparent viscosity decreases (µapp) decreases with rate of deformation and hence pseudo plastic fluids are also called as shear thinning fluids. Examples of dilatant fluid: Blood, Milk, colloidal solution and paper pulp in water. Case-3 Bingham Plastic (Ideal plastic) For Bingham plastic fluids, n=1 and τs ≠ 0 du τ = τs + C dy n du dy τ = τs + C du dy τ = τs + µapp where µapp is constant and does not vary with rate of deformation. Examples of Bingham Plastic fluid: Tooth paste and Gel Case -4 Thixotropic fluids These fluids show variation of viscosity with time. In these fluids apparent viscosity decreases with time. For Bingham plastic fluids, n 1 and τs ≠ 0 du dy τ = τs + µapp Examples of Thixotropic fluids: Paints, lipstick, crude oil and printers Ink Case-5 Rheopectic fluids These fluids also show variation of viscosity with time. Apparent viscosity of rheopectic fluids increases with time. For Bingham plastic fluids, n 1 and τs ≠ 0 du dy τ = τs + µapp Examples of Rheopectic fluids: Gypsum solution in water. Ideal fluids A fluid is said to be an ideal fluid if it is non-viscous, possess no cohesive forces and incompressible. From equation (3) du τ = µ dy for ideal fluid µ= 0, hence τ=0 Shear stress is zero in ideal fluids as viscosity is zero. Bulk modulus is ∞ for ideal fluid. Note: After deformation some fluids partially return to their original shape when applied stress is removed such fluids are called viscoelastic fluids. Examples: Solid-liquid combinations, Sewage. References 1. Fluid mechanics by Modi and Seth 2. Fluid mechanics by RK Bansal