* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Periodic Table Trends Notes s4
Survey
Document related concepts
Transcript
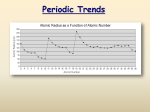
Periodic Table Trends Notes Topic Write questions here Write notes here How are periodic trends useful? Periodic Trends • The arrangement of the periodic table reveals trends in the properties of the elements. • Understanding a trend among the elements enables you to make predictions about the chemical behavior of the elements. What is the periodic trend in atomic radius? Atomic Radius Definition: measure of the size of atom, usually the distance from the nucleus to the boundary of the electron cloud Radius Radius decreases decreases across across aa period period from from left left to to right right Radius Radius increases increases down down aa group group Each Each row row down down on on the the periodic periodic table table adds adds an an energy energy level level to to the the atom atom making making the the radius radius bigger bigger Table of Atomic Radii Period Trend: Atomic Radius What is the periodic trend in Ionization Energy? Ionization Energy Definition: the amount of energy required to remove an electron from an atom and make it a positive ion. Tends Tends to to increase increase across across aa period period As As radius radius decreases decreases across across aa period, period, the the electron electron you you are are removing removing is is closer closer to to the the nucleus nucleus and and harder harder to to remove remove Tends Tends to to decrease decrease down down aa group group Outer Outer electrons electrons are are farther farther from from the the nucleus nucleus and and easier easier to to remove remove Periodic Trend of Ionization Energy Periodic Trend: Ionization Energy What is the periodic trend in Electronegativity? Electronegativity Definition: A measure of the ability of an atom in a chemical compound to attract electrons of the other atoms in an electron •• Electronegativity Electronegativity tends tends to to increase increase across across aa period period (Group (Group 18 18 does does not not bond bond && has has no no electronegativity) electronegativity) oo Electronegativity Electronegativity tends tends to to decrease decrease down down aa group group or or remain remain the the same same Periodic Trend: Electronegativity Summary of Periodic Trends What is the periodic trend in Ionic Radii? Ionic Radii Positively charged ions formed when an atom of a metal loses one or Cations more electrons Smaller than the corresponding atom Negatively charged ions formed when nonmetallic atoms gain one Anions or more electrons Larger than the corresponding atom Graphic courtesy Wikimedia Commons user Popnose Periodic Table Trends Review Key Ideas: The location of an element on the periodic table can be used to determine certain properties. The elements with the largest radii are in the bottom left corner. The elements with the largest ionization energy are in the top right corner. The elements with the highest electronegativity are in the top right corner excluding group 18. Terms to Know: electronegativity ionic radius atomic radius ionization energy