* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Module 6 Chemical Reactions
Nuclear fission wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Isotopic labeling wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear transmutation wikipedia , lookup
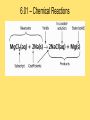
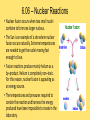
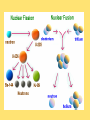
Module 6 Chemical Reactions 6.01 – Chemical Reactions • A chemical reaction, or chemical change, occurs when one or more substances react and change into completely different substances. • Signs of a Chemical Reaction • Formation of a new substance with properties different from its components. • a change in color is often an indication that a chemical reaction has occurred • the production of a gas from solids or liquids • the release of energy in the form of heat, light, or both. 6.01 – Chemical Reactions • Chemists use statements called equations to represent chemical reactions • Parts of a Chemical Reaction • Reactants – starting substances • Products – substances formed in the reaction • Subscripts – small numbers showing how many atoms of each element • Coefficients -a whole number placed in front of a formula in a chemical equation. 6.01 – Chemical Reactions 6.01 – Chemical Reactions • Recall, that matter cannot be created nor destroyed during a chemical reaction. • Chemists use coefficients to satisfy the law of conservation of mass. They add coefficients to reactants and products in a chemical equation until there is the same number of each kind of atom on both sides of the equation. • This is called balancing chemical equations. 6.01 – Chemical Reactions • Steps to balancing chemical equations 1.Create a T-chart and List the elements in the chemical formulas on each side in the SAME order 2.Count the number of atoms of each element on each side 3.See what elements don’t match in your T-chart 4.Add coefficients in front of one substance at a time, •Start by balancing the most complex compound first, •general rule of thumb is to save Hydrogen and Oxygen until the end to balance. 5.recount number of atoms of each element 6.Repeat 3, 4 & 5 until all elements are balanced. 6.01 – Chemical Reactions •____ N2 + ____ H2 ____ NH3 6.01 – Chemical Reactions •____ NaF + ____ Br2 ____ NaBr + ____ F2 6.01 – Chemical Reactions • ____ AgNO3 + ____ Cu ____ Cu(NO3)2 + ____ Ag 6.01 – Chemical Reactions •____ CH4 + ____ O2 ____ CO2 + ____ H2O 6.02 – Classifying Chemical Reactions • Chemical reactions take place everywhere • chemical reactions according to their similar properties. • One way to categorize chemical reactions is to examine the characteristics of the reactants and products. • six main categories: • • • • • • combustion reactions synthesis reactions decomposition reactions, single replacement reactions double replacement reactions acid–base reactions. 6.02 – Classifying Chemical Reactions • In a combustion reaction, a substance combines with oxygen and releases energy in the form of heat and light. We use combustion reactions every day to produce energy. • This type of reaction can produce enough energy to send a shuttle into space. • Most combustion reactions occur between oxygen and an organic compound C6H12O6 + 6O2 → 6CO2 + 6H2O + energy 6.02 – Classifying Chemical Reactions •In a synthesis reaction, two or more reactants combine to form one new product. Chemists often use the following generic equation to represent a synthesis reaction: •A + B → AB 2Cu + O2 → 2CuO 6.02 – Classifying Chemical Reactions •In a decomposition reaction, one reactant breaks down to form two or more products. •AB → A + B •a decomposition reaction is the reverse of a synthesis reaction. 2H2O → 2H2 + O2 6.02 – Classifying Chemical Reactions • In a single–replacement reaction, one element replaces another element in a compound to form a new substance. • Replacement reactions are also called displacement reactions. • A + BC → AC + B Cu + 2AgNO3 → Cu(NO3)2 + 2Ag 6.02 – Classifying Chemical Reactions • In a double–replacement reaction, the ions of two different compounds in an aqueous solution exchange places to form two new compounds. • The other product usually remains dissolved in the solution. The general form for a double-replacement reaction is shown below. • AC + BD → AD + BC Pb(NO3)2 (aq) + 2KI(aq) → 2KNO3(aq) + PbI2(s) 6.02 – Classifying Chemical Reactions • In an acid–base reaction, an acid and a base react to form salt and water. (also called a neutralization reaction) HCl + NaOH → NaCl + H2O acid + base → salt + water • We can rewrite the equation to show the ions involved in this reaction. H+ + Cl− + Na+ + OH− → Na+ + Cl− + H+ + OH− • Remember that most acids contain H+ and most bases contain OH−. HCl + NaOH → NaCl + H2O 6.03 – Chemical Reactions and Energy • Energy comes from chemical reactions. • Potential energy is stored in the chemical bonds in compounds. • When a chemical reaction occurs, some chemical bonds break, and others form. 6.03 – Chemical Reactions and Energy • Recall the law of conservation of energy states that energy cannot be created or destroyed. Energy can only change forms. • the reactants and products in a chemical reaction have different amounts of energy. • If the products have more energy than the reactants. The law of conservation of energy states that energy cannot be created out of nothing. So, the extra energy in the products must have come from somewhere in the environment. 6.03 – Chemical Reactions and Energy • All chemical reactions either release or absorb energy. Chemists classify chemical reactions into two groups based on this observation: -Exothermic Reactions -Endothermic Reactions 6.03 – Chemical Reactions and Energy • Characteristics of an exothermic reaction • Chemical reactions that result in a net release of energy. • The products are lower in energy than the reactants. • The excess energy is released in the form of heat and/or light as the reaction proceeds. • The rusting of metal is an exothermic reaction. It occurs so slowly that we don’t notice the heat being released. 6.03 – Chemical Reactions and Energy • An endothermic reaction absorbs heat from its environment as a way to make this happen. • Endothermic reactions result in products that have more energy than the reactants. • Photosynthesis is an example of an endothermic reaction. 6.03 – Chemical Reactions and Energy • Exothermic reactions produce the majority of heat — and light. • Heat is produced in these reactions and then transferred to the environment in three basic ways: • radiation – energy as waves or particles • conduction – direct contact of particles • convection – movement of particles 6.03 – Chemical Reactions and Energy • Recall that the kinetic molecular theory states that particles of matter are in constant motion. • For a chemical reaction to occur, the reactant particles have to come in contact with each other. • The rate at which a chemical reaction proceeds depends on three main factors: • Temperature • Concentration • Catalysts and inhibitors 6.03 – Chemical Reactions and Energy • An increase in temperature will increase the rate of reaction. A decrease in temperature will decrease the rate of reaction. 6.04 – Reaction Rates and Temperature • Concentration is a measure of the amount of a substance in a mixture. • An increase in concentration will increase the rate of reaction. A decrease in concentration will decrease the rate of reaction. 6.04 – Reaction Rates and Temperature • a catalyst can be used to increase the rate of a reaction. • A catalyst is a substance that speeds up a reaction, but does not itself participate in the reaction. • Catalysts work by lowering the activation energy - initial energy needed to begin a chemical reaction. • Biological catalysts called enzymes control the rates of chemical reactions in living things. 6.04 – Reaction Rates and Temperature • An inhibitor is a substance that slows down a chemical reaction, but is not itself consumed during the reaction. • Preservatives are inhibitors that are used in the food industry to slow down reactions that lead to food spoilage. • Catalysts increase the rates of reactions. Inhibitors decrease the rates of reactions 6.05 – Radioactivity • Remember that atoms are made up of smaller particles: protons, neutrons, and electrons. • The number of protons in an atom nucleus is its atomic number. Every element has a unique atomic number. • In addition to protons, an atom's nucleus also contains neutrons. The number of neutrons can vary between atoms of a given element. • Scientists use a special shorthand notation to represent different isotopes of an element. 6.05 – Radioactivity • Not all Atoms are Created Equal • The nucleus contains protons and neutrons. • The number of protons in an atom of an element is the same for each atom of that element. • all atoms of the same element do not contain the same number of neutrons. 6.05 – Radioactivity • Isotopes are atoms of the same element that have different numbers of neutrons. The carbon atoms mentioned here are called carbon–11, carbon–12, carbon–13, and carbon–14. The number tells you the total number of protons and neutrons in the nucleus. 6.05 – Radioactivity • Protons and neutrons are packed tightly in the nucleus and are held together by strong nuclear force. • The presence of neutrons in the nucleus contributes to strong nuclear force and helps hold the nucleus together. More protons in the nucleus, the more neutrons are needed balance the repulsive forces. • Unstable nuclei can break down into more stable nuclei in the process of radioactive decay 6.05 – Radioactivity • When an unstable nucleus releases and proton, the atomic number of the nucleus changes. If the atomic number changes, the atom of one element becomes an atom of a different element. • This process is called transmutation. • An unstable nucleus can emit different kinds of particles to become stable: alpha particles, beta particles, and gamma rays. 6.05 – Radioactivity • An alpha particle is a particle emitted from the nucleus during certain kinds of radioactive decay. • Alpha particles are positively charged & made up of two protons and two neutrons; they are also referred to as helium nuclei • alpha particles can be stopped by a sheet of paper • As positively charged alpha particles travel through air, they attract electrons, slow down quickly, and become harmless helium atoms. • if alpha particles are inhaled, swallowed, or injected into the bloodstream, they can damage living tissue 6.05 – Radioactivity • Emission of Alpha Particle • Loses 2 protons and 2 neutrons (alpha = helium nuclei) • Atomic Mass will decrease by 4 • Atomic Number will decrease by 2 Alpha Decay Practice 210 84 222 86 238 90 Po Rn Th 6.05 – Radioactivity • A beta particle is an electron emitted from the nucleus during certain kinds of radioactive decay. • An unstable neutron breaks apart to form a proton and an electron. The electron is released from the nucleus along with a large amount of energy. The proton remains in the nucleus. • Beta particles move faster in air than alpha particles and are harder to stop. Can pass through materials such as paper and clothing, penetrate skin and pass fairly deeply into the human body. Cannot penetrate denser materials, such as aluminum. 6.05 – Radioactivity • Emission of Beta Particle • Unstable neutron breaks apart to form a proton and an electron • Atomic Mass will stay the same • Atomic Number will increase by 1 Beta Decay Practice 14 C 6 40 19 K 90 Sr 38 6.05 – Radioactivity • Some transmutation processes (emitting alpha or beta) leave the nucleus in an excited state. The excited nucleus emits energy in the form of gamma rays. • Gamma rays, like visible light, are a form of electromagnetic radiation. They have no mass or charge. Gamma rays can be released along with alpha or beta particles during radioactive decay. • Gamma rays have no mass. They are made entirely of energy and can penetrate most materials. Very dense materials, such as lead, are able to stop gamma rays. Gamma rays are more harmful to humans than alpha or beta particles. 6.05 – Radioactivity • Emission of Gamma • transmutation processes (emitting alpha or beta) leave the nucleus in an excited state. • The excited nucleus emits energy in the form of gamma rays. • Atomic Mass will stay the same • Atomic Number will stay the same 6.05 – Radioactivity • Naturally Occurring Radiation • low levels of radiation from the natural environment (background radiation) • The Sun emits small amounts of gamma radiation and other kinds of harmful radiation. • Rocks and soil naturally contain small concentrations of radioactive elements, such as uranium. • Some kinds of rock release radon, a radioactive gas. Radon gas can be dangerous to people if it becomes concentrated in a small area. 6.05 – Radioactivity • Artificial Sources of Radiation • X–rays used to diagnose tooth decay and other diseases. • Radiation therapy is another source of radiation. • Some radioactive materials are used as tracers to diagnose cancer and other diseases. • Radioactive tracers are also used in agriculture to measure the amount of fertilizer used by the plant. From these measurements, they can tell farmers just how much fertilizer to use on their crops. 6.06 – Nuclear Reactions • In the late 1930s, scientist discovered that during a nuclear reaction - A reaction that changes the number of protons or neutrons in the nucleus of an atom • Today, nuclear energy is used as an alternative energy source and in the future may be used to replace some common resources such as natural gas, oil, and coal. • There are two main kinds of nuclear reactions: • nuclear fission • nuclear fusion. 6.06 – Nuclear Reactions • Nuclear fission, or radioactive decay, • when a heavy atomic nucleus splits into smaller nuclei. • For example, atoms of uranium–235 can break down into smaller particles. Some of those particles are atoms of lighter elements. The rest are neutrons. • During a nuclear reaction, matter changes into energy. 6.06 – Nuclear Reactions 2 • Einstein's famous equation, E = mc , shows how matter and energy are related. • In this equation, E stands for energy, m stands for mass, and c is the speed of light. (Remember that the speed of light is about 3 × 108 m ⁄ s.) • a small amount of mass is equivalent to an enormous amount of energy. • For example, if you converted just 1 g of matter completely into energy, you would get 9 × 1013 joules of energy. That is an amount of energy equivalent to about 22 billion kilocalories. 6.06 – Nuclear Reactions • If there are enough nuclei packed close together, the neutrons released by each fission can strike other nuclei; this is called a chain reaction. • As in a nuclear reactor or a nuclear bomb, there are many more millions of uranium nuclei, so more reactions that can be triggered. • In a nuclear reactor, the chain reaction is controlled. Devices called control rods are inserted into the reactor to absorb some of the neutrons. This reduces the number of reactions that occur and controls the amount of energy the reactor produces. In a nuclear bomb, the chain reaction is uncontrolled. All of the energy is released at once. 6.06 – Nuclear Reactions • Nuclear power plants can generate large amounts of electricity without producing any air pollution or greenhouse gases. • The waste products of nuclear reactions remain toxic for thousands of years, and they must be stored in special containers to prevent them from leaking into water and soil. • The disposal of nuclear waste is one of the main challenges to using nuclear fission to generate electricity. 6.06 – Nuclear Reactions • In April 1986, the nuclear power plant in Chernobyl, Ukraine, exploded. Almost 5 million people were forced to evacuate and 56 people died as a direct result of this accident. Several more thousand cancer deaths may also be attributable to Chernobyl, although it is difficult to assign these directly. • There is a big debate over the use of nuclear power. Some cite accidents such as Chernobyl as evidence that nuclear energy is too risky. Still others insist that accidents can be averted using strict safety measures, making nuclear energy one of the cleanest, safest energy options we have. 6.06 – Nuclear Reactions • Nuclear fusion occurs when two small nuclei combine to form one larger nucleus. • The Sun is an example of a site where nuclear fusion occurs naturally. Extreme temperatures are needed to get the nuclei moving fast enough to fuse. • Fusion reactions produce mainly helium as a by–product. Helium is completely non–toxic. For this reason, nuclear fusion is appealing as an energy source. • The temperatures and pressures required to contain the reaction and harness the energy produced have been impossible to create in the laboratory. 6.06 – Nuclear Reactions • It is important to understand the differences between chemical reactions and nuclear reactions.. •part of the atom involved in reactions. •amount of energy involved in each reaction. •the masses of the reactants and products.





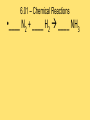


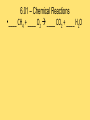






















































![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-150x150.png)

