* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Complimentary Slide Presentation.
Survey
Document related concepts
Transcript
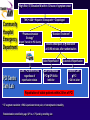
Trial of Routine ANgioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction The TRANSFER-AMI trial Warren J. Cantor, David Fitchett, Bjug Borgundvaag, Michael Heffernan, Eric A. Cohen, Laurie J. Morrison, John Ducas, Anatoly Langer, Shamir Mehta, Charles Lazzam, Brian Schwartz, Vladimir Dzavik, Amparo Casanova, Paramjit Singh, Shaun G. Goodman on behalf of the TRANSFER-AMI Investigators 9803mo01, 1 Trial Sponsors Canadian Institute of Health Research (CIHR) Hoffman LaRoche, Canada Stents provided by Abbott Vascular Canada Disclosures Consulting Fees & Speakers Honoraria received by Hoffman Laroche 9803mo01, 2 Background Treatment delays can reduce or eliminate benefits of primary PCI STEMI pts presenting to non-PCI centres often cannot undergo primary PCI in timely manner, and therefore receive thrombolysis The role and optimal timing of routine early PCI after fibrinolysis remains controversial 9803mo01, 3 Objective To compare: Pharmacoinvasive strategy (transfer to PCI centre for routine early PCI within 6 hrs) with Standard treatment (early transfer only for failed reperfusion, otherwise cath > 24 hrs) for high-risk STEMI patients receiving thromboysis at non-PCI centres. 9803mo01, 4 ‘High Risk’ ST Elevation MI within 12 hours of symptom onset Community Hospital Emergency Department TNK + ASA + Heparin / Enoxaparin + Clopidogrel “Pharmacoinvasive Strategy” Urgent Transfer to PCI Centre “Standard Treatment” Assess chest pain, ST resolution at 60-90 minutes after randomization Failed Reperfusion* PCI Centre Cath Lab Cath / PCI within 6 hrs regardless of reperfusion status Successful Reperfusion Cath and Rescue PCI GP IIb/IIIa Inhibitor Elective Cath PCI > 24 hrs later Repatriation of stable patients within 24 hrs of PCI * ST segment resolution < 50% & persistent chest pain, or hemodynamic instability Randomization stratified by age (≤75 vs. > 75) and by enrolling site 9803mo01, 5 Inclusion Criteria Within 12 hrs of symptom onset ≥ 2 mm ST-segment elevation in 2 anterior leads OR ≥ 1 mm ST-segment elevation in 2 inferior leads and at least one of the following: SBP < 100 HR > 100 Killip Class II-III ≥ 2mm ST-segment depression in anterior leads ≥ 1 mm ST-segment elevation in V4R 9803mo01, 6 Selected Exclusion Criteria Cardiogenic Shock PCI within 1 month Previous CABG Primary PCI available with DTB < 60 minutes Use of Enoxaparin in last 12 hours in patient > 75 years of age Consent not obtained within 30 minutes of TNK 9803mo01, 7 PCI for Pharmacoinvasive Group PCI of culprit lesion at time of cath if ≥ 70% stenosis or 50-70% stenosis with high-risk features (thrombus, ulceration, spont dissection) regardless of coronary flow Stents used whenever technically possible, use of Abbott vascular stents (ML Vision, Mini Vision) encouraged GP IIb/IIIa inhibitors left to operator’s discretion 9803mo01, 8 Endpoints 1o Efficacy Endpoint: 30-day composite of Death, Reinfarction, Recurrent Ischemia, CHF, shock * 2o Efficacy Endponts: Death / Reinfarction at 6 months and 1 Year Safety Endpoints: Bleeding (GUSTO Severe, TIMI Major) Endpoints adjudicated by clinical events committee blinded to treatment group 9803mo01, 9 * Endpoint definitions – Cantor WJ, Am Heart J 2008; 155: 19-25 Baseline Characteristics Age (years) Age > 75 (%) Sex (% female) Medical History (%) Prior Angina Prior MI Prior PCI Prior Stroke/TIA * Hypertension Hyperlipidemia Current smoker Diabetes Standard Treatment (n=508) Pharmacoinvasive Strategy (n=522) 56 (49, 66) 10 20 57 (51, 66) 9 21 11 10 4 1 34 29 42 15 12 11 6 3 33 27 44 15 * p< 0.05 9803mo01, 10 Presenting Characteristics Standard Pharmacoinvasive Treatment Strategy (n=508) (n=522) 80 (70, 91) 80 (70, 91) 77 (66, 90) 74 (63, 88) 145 (130, 160) 146 (130, 165) 84 (74, 95) 84 (73, 95) Weight (kg) Heart rate (beats/min) Systolic BP (mm Hg) Diastolic BP (mm Hg) Killip Class I 91 II 7 III 1 Anterior ST-elevation 52 Inferior ST-elevation 47 Symptom Onset to TNK (hrs) 2 (1, 3) 92 7 1 56 44 2 (1, 3) 9803mo01, 11 Procedures Standard Treatment (n=508) Cardiac Cath performed (%) 82 Time- TNK to Cath (hrs) 27 (4, 69) PCI performed (%) 62 Stent used (% of PCI cases) 98 Time- TNK to PCI (hrs) 18 (4, 73) PCI within 6 hrs of TNK (%) 38 PCI within 12 hrs of TNK (%) 47 GP IIb/IIIa inhibitor use (%) 53 Time- TNK to GP IIb/IIIa inhib. (hrs) 11 (4, 63) IABP use (%) 6 CABG performed (%) 8 Pharmacoinvasive Strategy (n=522) 97 3 (2, 4) 84 98 4 (3, 5) 89 97 73 4 (3, 5) 7 6 9803mo01, 12 Selected Medications Used Standard Treatment (n=508) ASA 1st 6 hrs 97 Clopidogrel 1st 6 hrs * 69 Heparin 57 Enoxaparin 55 Beta Blocker 1st 6 hrs 61 ASA at discharge 85 Clopidogrel at discharge 73 Beta Blocker at discharge 79 ACE Inhibitor at discharge 74 Lipid Lowering at discharge 80 Pharmacoinvasive Strategy (n=522) 98 87 57 51 55 85 79 81 73 81 * p< 0.05 9803mo01, 13 Primary Endpoint: 30-Day Death, re-MI, CHF, Severe Recurrent Ischemia, Shock % of Patients 18 16.6 16 14 OR=0.537 (0.368, 0.783); p=0.0013 12 10.6 10 8 6 4 2 0 Standard (n=496) Pharmacoinvasive (n=508) 0 5 n=496 n=508 422 468 10 15 20 Days from Randomization 415 466 415 463 414 461 25 30 414 460 412 457 9803mo01, 14 Components of Primary Endpoint Death Reinfarction Recurrent Ischemia Death/MI/Ischemia New / worsening CHF Cardiogenic Shock Standard Treatment (n=498) 3.6 6.0 2.2 11.7 5.2 2.6 Pharmacoinvasive Strategy P-Value (n=512) 3.7 0.94 3.3 0.044 0.2 0.019 6.5 0.004 2.9 0.069 4.5 0.11 9803mo01, 15 Safety Endpoints - Bleeding Standard Treatment (n=498) Intracranial hemorrhage TIMI scale Major Major (non-CABG-related) GUSTO scale Moderate Severe Severe (non-CABG-related) Transfusions Pharmacoinvasive Strategy P-Value (n=512) 1.2 0.2 0.066 4.6 3.2 4.3 2.2 0.88 0.33 2.2 1.4 1.2 5.5 3.5 0.6 0.6 7.1 0.26 0.22 0.34 0.31 9803mo01, 16 Summary Compared with ‘Standard Treatment’, a ‘Pharmacoinvasive Strategy’ of routine early PCI within 6 hrs after thrombolysis is associated with a 6% absolute (46% relative) reduction in the composite of death, reinfarction, recurrent ischemia, heart failure and shock The pharmacoinvasive strategy is not associated with any increase in transfusions, severe bleeding or intracranial hemorrhage despite high use of GP IIb/IIIa inhibitors during PCI In contrast to older trials, routine early PCI after thrombolysis using stents and contemporary pharmacotherapy is safe and effective Benefit seen despite high cath/PCI rates in Standard Treatment group (including ~40% rescue PCI) 9803mo01, 17 Conclusions For high-risk STEMI patients receiving thrombolysis at non-PCI centres, urgent transfer and PCI within 6 hours is associated with significantly less ischemic complications and no excess in bleeding Transfers to PCI centres should be initiated immediately after thrombolysis without waiting to see whether reperfusion is successful Regional systems should be developed to ensure timely transfers of STEMI patients to PCI centres 9803mo01, 18