* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 7 Thyroid Gland
Survey
Document related concepts
Transcript
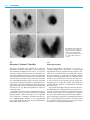
Chapter 7 Thyroid Gland 7 Salil D. Sarkar 7.1 Thyroid Anatomy 209 7.2 7.2.1 7.2.2 7.2.3 7.2.4 7.2.5 7.2.6 Hormone Synthesis and Secretion 210 Iodide Transport 210 Hormone Synthesis 210 Release of Hormone and Thyroglobulin 210 T3 and T4 210 Antithyroid Drugs 210 Summary 211 7.3 7.3.1 7.3.2 7.3.3 7.3.4 7.3.5 Thyroid Handling of Radiotracers 211 Technetium-99m-pertechnetate 211 Iodine-123 211 Iodine-131 211 Fluorine-18-fluorodeoxyglucose 212 Summary 212 7.4 7.4.1 7.4.2 7.4.3 7.4.3.1 7.4.3.2 7.4.4 TSH and Thyroid Function 212 TSH Secretion 212 Serum TSH in Thyroid Disorders 212 Manipulation of TSH Levels 213 Suppressing TSH Levels 213 Increasing TSH Levels 213 Summary 213 7.5 7.5.1 7.5.2 7.5.2.1 7.5.2.2 7.5.2.3 7.5.3 Iodine Intake and Thyroid Function 213 Iodine Deficiency 213 Iodine Excess 214 Thyroid Autoregulation 214 Thyroid Dysfunction 214 Iodine and Autoimmune Thyroid Disease 214 Summary 214 7.6 7.6.1 7.6.2 7.6.3 7.6.4 Endemic Goiter 215 Goitrogens 215 Pathophysiology 215 Radionuclide Procedures Summary 215 7.7 7.7.1 7.7.2 7.7.3 7.7.4 7.7.5 Destructive (“Subacute”) Thyroiditis 216 Postpartum Thyroiditis 216 Viral Thyroiditis 217 Thyroiditis and Other Effects of Amiodarone 217 Radionuclide Procedures 217 Summary 217 7.8 7.8.1 7.8.2 7.8.3 7.8.4 Autoimmune Thyroid Disease 217 Etiological Factors 217 Pathophysiology 218 Radionuclide Procedures 218 Summary 218 7.9 7.9.1 7.9.2 7.9.3 Thyroid Dysfunction During Gestation Hyperthyroidism 218 Hypothyroidism 219 Summary 219 References 219 7.1 Thyroid Anatomy The thyroid gland develops from the foramen cecum of the tongue, to which it is connected by the thyroglossal duct. It descends during fetal life to reach the anterior neck by about the seventh week, and absent or aberrant descent results in ectopic locations, including the sublingual region and superior mediastinum (Fig. 7.1). The thyroglossal duct undergoes atrophy, though remnant duct tissue frequently is visualized by scintigraphy as an upper midline neck structure following thyroidectomy and TSH stimulation. The duct remnant occasionally may form a cyst. The normal adult thyroid gland in iodine-sufficient regions weighs about 14 – 18 g. It is generally smaller in women than in men, and is barely palpable [1, 2]. The thyroid is located in the mid to lower anterior neck, with the isthmus in front of the trachea, usually just below the cricoid cartilage, and the lobes on the sides of the trachea. In older individuals with shorter necks, the thyroid may lie at or just above the suprasternal notch, and it is often partly substernal. The thyroid gland moves cephalad during swallowing, a characteristic that aids in palpation and in distinction of thyroid from nonthyroid neck masses. 215 218 Fig. 7.1. Scintigraphic images in the anterior and left lateral projections show partly descended thyroid gland extending from the sublingual region to the upper neck 210 7 Thyroid Gland 7.2 Hormone Synthesis and Secretion 7.2.1 Iodide Transport The thyroid follicle consists of a colloid center, which acts as a storage site for thyroid hormone, surrounded by epithelial cells. The thyroid epithelial cell has a transport mechanism, also referred to as “trapping” or “uptake”, that enables thyroid concentration of iodide far in excess of that in the plasma [3, 4]. A plasma membrane protein, the sodium/iodide symporter (NIS), is responsible for iodide transport. Symporter activity is influenced primarily by pituitary thyrotropin, also called thyroid stimulating hormone (TSH), which increases the transport of iodide. The trapped iodide subsequently undergoes organification and incorporation into thyroid hormones. Iodide is accumulated, though not organified, in other organs including the salivary glands, stomach, mucous glands, skin, breast, and placenta, which may be associated with undesirable consequences for the clinical use of radioiodine. After therapeutic administration of 131I for thyroid cancer, uptake in the salivary glands and gastric mucosa may cause sialitis and gastritis respectively, while activity in the skin and mucous secretions may increase environmental contamination and interfere with image interpretation [5 – 7]. Iodide uptake by the placenta and mammary glands exposes the fetus and the nursing child to unacceptable amounts of radiation from both the therapeutic and diagnostic use of 131I [8, 9]. Other anions, including pertechnetate, thiocyanate, and perchlorate, also are accumulated by the thyroid gland. The uptake of pertechnetate is the basis for 99m Tc-pertechnetate scintigraphy. Thiocyanate, derived from certain foods, decreases thyroid accumulation of iodine and may exacerbate iodine deficiency. Perchlorate has diagnostic and therapeutic applications, which are discussed later. 7.2.2 Hormone Synthesis Iodide transported via NIS at the basolateral cell membrane is converted to an oxidized form at the apical surface of the cell by thyroid peroxidase (TPO) in the presence of hydrogen peroxide. Oxidation of iodide permits its binding to the amino acid tyrosine. Synthesis of hormone takes place in thyroglobulin, a glycoprotein, which is produced in the thyroid cell and extruded into the colloid. Iodine combines with tyrosine in thyroglobulin to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). Subsequently, the iodotyrosines are coupled, with the formation of thyroxine (T4) and triiodothyronine (T3). The coupling reaction also is mediated by peroxidase. Decrease in peroxidase, associated with certain congenital and acquired thyroid disorders, impairs organic iodination and increases the proportion of unbound intrathyroidal iodine. Potassium perchlorate in pharmacological doses discharges unbound iodine from the thyroid. This is the basis for its use in the “Perchlorate Discharge Test” to detect an organification defect [10 – 12], and in the treatment of thyroid dysfunction caused by amiodarone, an iodine-rich drug (see later). 7.2.3 Release of Hormone and Thyroglobulin In response to TSH, a small amount of colloid is engulfed by the epithelial cell and proteolyzed, with release of T3 and T4, which diffuse into the circulation. Thyroglobulin not undergoing proteolysis also enters the circulation in small quantities. The serum thyroglobulin has been used as a tumor marker in differentiated thyroid cancer. Thyroglobulin becomes undetectable following thyroidectomy and 131I ablation, and its subsequent rise indicates a recurrence. TSH stimulation, by promoting colloid endocytosis, increases the amount of thyroglobulin released. Consequently, the serum thyroglobulin is a more reliable tumor marker at high TSH levels [13, 14]. 7.2.4 T3 and T4 Most of the circulating thyroid hormones are bound to plasma proteins, the free fraction comprising about 0.05% of T4 and 0.2% of T3. Only the free hormone has metabolic effects, and it is a more accurate measure of thyroid function than the total hormone, which varies with plasma proteins levels. T3 is considered the active hormone. About 20% – 30% of the circulating T3 is secreted by the thyroid gland and the remainder is produced by monodeiodination of T4 in extrathyroid tissues, notably the liver, kidney, brain, and pituitary [14]. Decrease in the peripheral conversion of T4 to T3 is a basis for the use of some antithyroid drugs (see below). Synthetic forms of thyroid hormones are commonly used for replacement and/or suppressive therapy. Thyroxine is preferred for this purpose because it has a longer biological half-life (6 – 7 days) compared with T3 (about 1 – 2 days). However, T3 has a more rapid onset of action and may be useful in selected clinical situations. 7.2.5 Antithyroid Drugs Most antithyroid drugs generally block one or more steps in the synthesis and metabolism of thyroid hor- 7.3 Thyroid Handling of Radiotracers mone. The thiourea derivatives (“thionamides”), including propylthiouracil (PTU) and methimazole, are the most common antithyroid agents in use [14, 15]. Both decrease hormone synthesis primarily by blocking iodine organification, while PTU alone decreases the monodeiodination of T4 to T3. These drugs also lower serum levels of thyrotropin receptor autoantibodies (TRAB), which are responsible for Graves’ hyperthyroidism. Methimazole or PTU may be used to control hyperthyroidism in Graves’ disease and toxic nodular goiter before treatment with 131I. In selected patients, these drugs also may be used as primary therapy for Graves’ disease. Remission occurs in a minority of patients after thionamide treatment for 1 – 2 years. Other drugs used for their antithyroid actions include glucocorticoids, iodides, lithium, and potassium perchlorate [14]. Glucocorticoids have a rapid inhibitory effect on the peripheral conversion of T4 to T3, and are a useful adjunct in thyroid storm. Their anti-inflammatory and cell membrane stabilizing actions have been utilized in Graves’ ophthalmopathy and protracted subacute thyroiditis. Iodide in pharmacological amounts decreases the synthesis of thyroid hormones, permitting rapid control of hyperthyroidism in thyroid storm (see “Thyroid Autoregulation”). It also blocks thyroid uptake of radioiodine, and is recommended as a prophylactic measure after a nuclear reactor accident [16]. Lithium blocks the release of thyroid hormone, and may be used as an adjunct for the control of severe hyperthyroidism. Lithium prolongs iodine retention in thyroid tissue, and increases the absorbed radiation dose from 131I, an advantage in the treatment of differentiated thyroid cancer [17]. Potassium perchlorate decreases thyroid iodine uptake and discharges unbound iodine. It may be used for the treatment of thyroid dysfunction caused by amiodarone, a drug with a high iodine content, and after accidental exposure to radioactive iodine. 7.2.6 Summary Synthesis and secretion of thyroid hormone are regulated primarily by thyrotropin. Circulating iodide is trapped by the thyroid epithelial cell, oxidized, and bound to tyrosine. Coupling of iodotyrosines yields T3 and T4. Thyroid peroxidase promotes oxidation of iodide, a necessary step for iodination of tyrosine, as well as coupling of iodotyrosines. Thyroid hormone action is mediated by T3. About 20% – 30% of the circulating T3 is secreted by the thyroid, and the remainder is derived from the peripheral monodeiodination of T4. Among the drugs with antithyroid actions, PTU and methimazole are most commonly used. Both drugs decrease hormone synthesis and TRAB levels, while PTU alone decreases the conversion of T4 to T3. 7.3 Thyroid Handling of Radiotracers 7.3.1 Technetium-99m-pertechnetate Technetium-99m-pertechnetate is widely used for imaging the thyroid gland [18, 19]. The popularity of this radiotracer stems from its easy availability (from portable molybdenum-99 generators) and low absorbed radiation dose (short half-life of 6 h and absence of beta emissions). 99m Tc-pertechnetate is trapped by the thyroid, but unlike iodine, it does not undergo organification and remains in the gland for a relatively short period. Therefore, imaging is done about 20 – 30 min after administration of the radiotracer. Approximately 5 – 10 mCi (185 – 370 MBq) is used. The thyroid-tobackground activity ratio is not as high as that with radioiodine, so that 99mTc-pertechnetate is unsuitable for imaging of metastatic thyroid carcinoma, which usually functions poorly compared with normal tissue. Imaging of ectopic mediastinal thyroid tissue also may be suboptimal due to high blood and soft tissue background activity. 7.3.2 Iodine-123 Iodine-123 has ideal characteristics for imaging the thyroid gland, with a short physical half-life of 13 h, absence of beta emissions, and high uptake in thyroid tissue relative to background. However, it is less readily available and more expensive than 99mTc-pertechnetate. 123I undergoes organic binding in the thyroid gland, and imaging is usually done 4 – 24 h after the administration of 200 – 400 µCi (7.4 – 14.8 MBq) of radiotracer [18, 19]. Because of its superior biodistribution characteristics, 123I is preferred over 99mTc-pertechnetate for imaging of poorly functioning and ectopic thyroid glands. 123I also may be used for whole body imaging in differentiated thyroid cancer (see below). Approximately 2 – 4 mCi (74 – 148 MBq) of the radiotracer are used for this purpose. 7.3.3 Iodine-131 Iodine-131 may be used for the measurement of thyroid uptake, which requires only small amounts of radiotracer. It is no longer used for routine imaging of the thyroid gland because of a high absorbed radiation dose related to the long physical half-life of 8 days and beta emissions. 131I, however, continues to be valuable for the detection of metastases and recurrences in differentiated thyroid cancer [13, 19, 20]. Following appropriate patient preparation to increase TSH levels 211 212 7 Thyroid Gland (see “Manipulation of Thyrotropin Levels”), 2 – 4 mCi (74 – 148 MBq) of 131I is administered and imaging is performed 48 – 96 h later. Radioiodine imaging has diagnostic as well as prognostic value. Iodine-avid tumors tend to have well-differentiated histological features and a favorable prognosis, whereas tumors that do not accumulate iodine are likely to be less differentiated and more aggressive [13, 21, 22]. Iodine-131 delivers a high radiation absorbed dose to the thyroid, with relative sparing of non-thyroid tissues. It is therefore ideal for the treatment of thyroid disease, and used extensively in the management of Graves’ disease, toxic nodular goiter, and differentiated thyroid cancer. 7.3.4 Fluorine-18-fluorodeoxyglucose Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is used in evaluating a variety of neoplasms including differentiated thyroid cancer. Imaging is possible for two reasons. First, malignant tumors derive energy from a higher rate of glycolysis, so that the uptake of glucose (and FDG) is increased. Second, unlike glucose, FDG is not metabolized completely and retained longer within the tumor. In differentiated thyroid cancer, FDG may be used to identify metastases not visualized at radioiodine imaging, and to assess prognosis. Lesions that accumulate FDG tend to follow a more aggressive course than lesions that are not FDG-avid [23, 24]. Whole body FDG-PET, therefore, is useful in evaluating high-risk thyroid cancer. Patient preparation is similar to that for radioiodine scintigraphy, since the uptake and diagnostic sensitivity of FDG are increased by TSH stimulation [25, 26]. Focal uptake of FDG within the thyroid gland, an occasional finding at evaluation of non-thyroid cancers, may be related to a benign or malignant pathology. 7.3.5 Summary 99mTc-pertechnetate is trapped but not organified by thyroid tissue. Imaging with this radiotracer is limited to the intact thyroid gland. 123I and 131I are trapped and organified, and provide higher thyroid-to-background uptake ratios. Both tracers are used to detect thyroid cancer metastases, while 123I is also used for imaging the thyroid gland. 131I delivers a high absorbed radiation dose to thyroid tissue, and is a mainstay in the management of Graves’ disease, toxic nodular goiter, and differentiated thyroid cancer. Imaging and treatment of thyroid cancer metastases with 131I require high TSH levels. 18F-FDG, a glucose analogue, is accumulated in various malignant tumors including differentiated thyroid cancer. FDG-PET is particularly useful in high risk thyroid cancer, where it may detect metastases not visualized at radioiodine imaging and provide prognostic information. Tumor uptake of FDG is increased by TSH stimulation. 7.4 TSH and Thyroid Function 7.4.1 TSH Secretion Thyrotropin-releasing hormone (TRH), a tripeptide originating from the hypothalamic median eminence, stimulates the secretion and synthesis of thyroid stimulating hormone (TSH, thyrotropin), a glycoprotein, by the anterior pituitary. TSH comprises an alpha unit, also present in other anterior pituitary hormones (FSH, LH), and a beta unit responsible for its specific actions. It acts on specific membrane-bound receptors of the thyroid epithelial cell, activating the adenylate cyclase system and increasing sodium/iodide symporter expression. As a result, the transport of iodide, synthesis of hormone, and release of T3, T4, and thyroglobulin are increased. The production and release of TSH are influenced by the concentration of T3 within the pituitary. When the T3 concentration falls below a “set point”, TSH secretion increases, and synthesis and release of thyroid hormones are accelerated. Conversely, when the T3 level rises above the set point, TSH release is inhibited. In addition to its pituitary effect, T3 inhibits hypothalamic TRH release. Other mechanisms reported more recently include the inhibitory actions of the released TSH on TRH secretion, and on TSH receptors in the pituitary itself. In sum, TSH secretion is influenced by thyroidto-pituitary, thyroid-to-hypothalamus, pituitary-tohypothalamus, and pituitary-to-pituitary feedback control mechanisms, which combine to reduce fluctuations in circulating T3 and T4 [14, 27 – 28]. In the rare condition of partial tissue resistance to thyroid hormone, the pituitary fails to respond to increasing T3 levels, so that TSH continues to be secreted and serum TSH and thyroid hormones are both elevated. Individuals with this condition may become hyperthyroid if tissue resistance is limited to the pituitary or remain euthyroid if resistance is generalized [29]. In addition to regulation of thyroid function, TSH promotes thyroid growth. If thyroid hormone synthesis is chronically impaired, as in iodine deficiency and autoimmune thyroid disease, chronic TSH stimulation eventually may lead to the development of a goiter. 7.4.2 Serum TSH in Thyroid Disorders The serum TSH is a sensitive marker of thyroid function. Normal serum TSH is about 0.45 – 4.5 µunits/ml, 7.5 Iodine Intake and Thyroid Function and levels up to 20 µunits/ml are considered normal in newborns because of the contribution of maternal TSH. During early gestation, TSH tends to be at low normal (at times below normal) levels, which coincide with a surge in human chorionic gonadotropin (hCG) release. Serum TSH is increased in primary hypothyroidism and decreased in hyperthyroxinemia of all etiologies except for the uncommon entity of thyrotropininduced hyperthyroidism. The availability of high sensitivity assays, which can accurately measure very low TSH levels, has significantly improved the ability to diagnose mild hyperthyroidism. Third-generation assays can detect levels as low as 0.01 – 0.03 µunits/ml and are particularly helpful in establishing subclinical hyperthyroidism in nodular goiter and athyrotic persons receiving replacement levothyroxine therapy [27, 30]. Subclinical hyperthyroidism in older individuals has been associated with adverse effects on the heart and bone mineral density [31 – 34]. The serum TSH is also a sensitive marker of hypothyroidism. As such it is commonly used to detect hypothyroidism in Hashimoto’s disease, newborns, and hyperthyroid patients treated with 131I. The TRH Stimulation Test measures the TSH response to TRH. It was used in the past for the diagnosis of subtle thyroid dysfunction including central hypothyroidism, but has been largely abandoned with the emergence of highsensitivity TSH assays [35]. 7.4.3 Manipulation of TSH Levels 7.4.3.1 Suppressing TSH Levels The secretion of TSH is suppressed with exogenous thyroid hormone to avoid stimulation of tumor growth in patients with differentiated thyroid cancer, and to decrease thyroid size or arrest thyroid growth in the early stages of goiter development. While levothyroxine (T4) is the traditional thyroid hormone preparation for this purpose, regimens combining T4 and T3 are currently under investigation. Not infrequently, patients receiving levothyroxine are referred for a nuclear uptake and scan, requiring hormone withdrawal to allow the recovery of the hypothalamus-pituitary-thyroid axis. It may take as long as 8 weeks for recovery and for return of radioiodine uptakes to baseline values; however, shorter periods of up to 3 weeks may suffice for evaluating nodular function. ment of thyroid remnants and thyroid cancer metastases with radioiodine [13, 19, 36]. Thyroid stimulating hormone levels are allowed to rise to 30 – 50 µunits/ml or higher after withholding thyroid hormone supplements, or after administering recombinant human TSH. The latter is gaining in popularity since it shortens the preparation time and avoids a period of hypothyroidism [37 – 41]. Currently, recombinant TSH is approved primarily for diagnostic use, i.e., prior to scintigraphy and serum thyroglobulin measurement. It appears to be effective in monitoring thyroid cancer, especially the lowrisk papillary type, though the radioiodine uptake and serum thyroglobulin usually are lower than after hormone withdrawal. As noted earlier, PET with fluorodeoxyglucose is optimal at high TSH levels, and it may be combined with radioiodine imaging and thyroglobulin measurement in selected patients [23 – 26]. Recombinant human TSH may have the potential to facilitate the treatment of large nodular goiters with 131 I. Radioiodine uptake in these goiters is usually low and heterogeneous. As a result, large and multiple therapeutic 131I doses may be needed to reduce goiter volume and cure the associated hyperthyroidism. In recent studies, a small dose of recombinant TSH resulted in a more uniform 131I distribution, a higher 24-h uptake, and increased therapeutic efficacy [42, 43]. 7.4.4 Summary Thyroid stimulating hormone (thyrotropin) promotes iodide transport, and the synthesis and release of thyroid hormone and thyroglobulin. The secretion of TSH is modulated by the hypothalamus-pituitary-thyroid axis. The serum TSH level is a sensitive and specific marker of primary hyperthyroidism and hypothyroidism, and is particularly valuable for diagnosing subclinical thyroid dysfunction. Suppression of TSH secretion with exogenous thyroid hormone may help reduce goiter size and limit the growth of thyroid cancer. In athyrotic patients with differentiated thyroid cancer, a high serum TSH is needed for radioiodine/FDG imaging, thyroglobulin measurement, and 131I treatment. The serum TSH may be increased by withdrawing thyroid hormone, or by administering recombinant human TSH. 7.5 Iodine Intake and Thyroid Function 7.4.3.2 Increasing TSH Levels 7.5.1 Iodine Deficiency Stimulation with TSH increases thyroid function and thyroid uptake of radioiodine. This principle is used in differentiated thyroid cancer for the detection and treat- The daily requirement for iodine is about 150 µg, increasing to roughly 200 – 250 µg during pregnancy. Iodine deficiency is most prevalent in the mountainous 213 214 7 Thyroid Gland regions of the Himalayas, Alps, and Andes, and in some low lands remote from the ocean. Iodine deficiency alone or in combination with goitrogens present in certain foods results in decreased thyroid hormone synthesis [44, 45]. Selenium deficiency may be a contributing factor. Reduced synthesis of thyroid hormone is compensated, at least in part, by increased TSH secretion, resulting eventually in goiter formation. Because an adequate supply of thyroid hormone is needed for fetal neurological development, maternal and fetal hypothyroidism resulting from iodine deficiency is associated with varying degrees of neuropsychological deficits including cretinism [46 – 50]. 7.5.2 Iodine Excess 7.5.2.1 Thyroid Autoregulation Thyroid hormone homeostasis is maintained by an intrathyroid autoregulatory mechanism in addition to the hypothalamus-pituitary-thyroid axis. When intrathyroid iodine concentrations are significantly increased, the rate of thyroid hormone synthesis is decreased, with a reduction in iodothyronine synthesis and decrease in the DIT/MIT ratio. This response is referred to as the Wolff-Chaikoff effect [51]. The amount of intrathyroid iodine needed to trigger the Wolff-Chaikoff effect varies, depending on prior long-term iodine intake and thyroid function. Barring other mechanisms, continued exposure to large amounts of iodine would eventually lead to hypothyroidism, with compensatory increase in TSH and development of goiter. While this does occur occasionally (see below), adaptation or “escape” from the effects of chronic iodide excess is more likely. Adaptation appears to be the result of an absolute decrease in iodide transport, so that intrathyroid iodine is reduced to levels that allow resumption of hormone synthesis. The inhibitory effect of iodides on thyroid function is utilized clinically for prompt control of severe hyperthyroidism and thyroid storm. In Graves’ disease, large doses of iodide decrease not only hormone synthesis but also hormone release [52]. Since escape from the inhibitory effect is likely, iodide therapy is only a shortterm measure for lowering thyroid hormone levels rapidly. 7.5.2.2 Thyroid Dysfunction Iodine excess may lead to hyperthyroidism or hypothyroidism [51 – 54]. Iodine-induced hyperthyroidism, referred to as jodbasedow, characteristically occurs in persons with nodular thyroid glands. Hyperthyroidism occurring after iodine supplementation in endemic goiter areas is a classical example. Iodine-containing medical products, including amiodarone, radiographic dyes, and kelp, also have the potential to cause jodbasedow [51, 55 – 58]. Amiodarone, a cardiac antiarrhythmic drug, is perhaps the commonest source of iodine today. Each 200 mg tablet yields about 7 mg free iodine, while the daily requirement is only 0.15 mg [55, 56]. Amiodarone-related hyperthyroidism may be related to another mechanism. The drug may cause thyroiditis, which is discussed later (see “Destructive (Subacute) Thyroiditis”]. Hypothyroidism related to increased iodine intake results from the inability to escape from the WolffChaikoff effect. It is more frequent in iodine-sufficient areas, where autoimmune disease is more common than nodular disease [53, 54]. In the past, “iodide goiter” with or without hypothyroidism was related to the use of iodine solutions as mucolytic agents in bronchial asthma, often with reversal of clinical manifestations after stopping the drug. A similar condition has been reported from ingestion of large quantities of (iodinerich) seaweed in the coastal regions of Japan [59]. 7.5.2.3 Iodine and Autoimmune Thyroid Disease Iodine appears to have another, more insidious effect on the thyroid. In regions that were previously iodinedeficient, a rise in autoimmune thyroid disease has been observed after the institution of iodine supplementation in foods [60]. Experimental work in animals confirms an association between iodine and autoimmunity, probably related in part to the greater antigenic potential of highly iodinated thyroglobulin [61]. Autoimmune thyroid disease and associated disorders are discussed under “Hashimoto’s Disease”. 7.5.3 Summary Excessive amounts of iodine may cause hypothyroidism or hyperthyroidism. A significant increase in thyroid concentration of iodine may initiate an autoregulatory response, the Wolff-Chaikoff effect, which decreases hormone synthesis. Although this effect is usually temporary, occasionally it may be sustained and lead to hypothyroidism. Iodine-induced hypothyroidism is more frequent in iodine-replete regions with a high prevalence of autoimmune thyroid disease. Excessive iodine also may lead to hyperthyroidism. This may occur in individuals with nodular thyroid glands, and it is more common in iodine-deficient areas. In addition to its effects on thyroid function, iodine is believed to promote the development of autoimmune thyroid disease, a view supported by epidemiological and experimental evidence. 7.6 Endemic Goiter 7.6 Endemic Goiter Endemic goiter is attributed primarily to iodine deficiency, possibly in association with selenium deficiency or goitrogens. Goitrogens are present in certain foods and chemicals and cause either decreased synthesis or increased metabolism of thyroid hormone. 7.6.1 Goitrogens Certain foods including cassava and bamboo shoots contain cyanogenic compounds, which may interfere with thyroid accumulation of iodine and exacerbate iodine deficiency [62]. Other foods with goitrogenic potential include pearl millet and plants from the brassica family [63]. Various chemicals may alter thyroid hormone metabolism and lead to the development of goiter. Contamination of drinking water with ammonium perchlorate from discarded rocket fuel is a concern. However, the suggested regulatory limit for perchlorate concentration in water is well below the amount needed to block iodine uptake [64]. Cigarette smoking has been linked to thyroid disease and aggravation of Graves’ ophthalmopathy. The effects presumably are mediated in part by thiocyanate [65]. Other industrial chemicals and drugs may induce hepatic enzymes that accelerate the metabolic elimination of thyroid hormone [66, 67]. 7.6.2 Pathophysiology The thyroid enlarges primarily in response to TSH stimulation resulting from inefficient hormone synthesis. There is natural heterogeneity in cellular growth and response to TSH, and rapid proliferation of thyrocytes with a growth advantage leads eventually to the development of nodules. An additional mechanism for nodule formation involves the activation of the adenylate cyclase system, usually by somatic mutations of the TSH receptor, with increase in cell replication rates [68 – 71]. Evidence of such mutations has been found in both solitary nodules and nodules associated with multinodular goiters. The development of toxic nodular goiter occurs over a period of years, if not decades, with gradual transition of cell clones to micronodules, and subsequently to macronodules of sufficient size to cause hyperthyroidism. The disorder, therefore, is typically seen in older individuals. Hyperthyroidism associated with nodular goiter is often subclinical, with a suppressed TSH and a normal free T4. Nonetheless, treatment with 131I or surgery is generally recommended in the elderly because of increased risk of osteopenia, and of adverse cardiovascu- lar sequelae including atrial fibrillation [31 – 34]. Suppressive levothyroxine therapy is often attempted to arrest nodular growth in euthyroid patients, but is rarely successful since the nodules are largely independent of TSH control [72]. Hyperfunctioning nodules may become “cold” or non-functional due to hemorrhage and necrosis. Cold nodules also may be caused by the failure of iodide transport with aging, rapid proliferation of cells with decreased function, and malignant transformation. 7.6.3 Radionuclide Procedures Toxic multinodular goiters typically show irregular distribution of radioiodine or technetium pertechnetate, and a normal or mildly elevated 24-h radioiodine uptake. The irregular tracer distribution is consistent with heterogeneity in cell function and growth, and the presence of micro- and macronodules (Fig. 7.2). Large and discrete hyperfunctioning nodules may be associated with poor uptake in the extranodular thyroid tissue. The latter consists of “suppressed” normal tissue, and/or small autonomous nodules with relatively less tracer accumulation. Following 131I treatment, the areas that were previously “cold” may appear more active. A dominant nonfunctioning nodule may be related to a number of causes, but may require additional diagnostic work-up to exclude malignancy. Nodular disease may be treated with 131I or surgery. For large multinodular goiters, the goal of 131I treatment is to reduce thyroid volume and cure hyperthyroidism. But the treatment may fail because radioiodine distribution is heterogeneous and the 24-h uptake is not significantly elevated. Stimulation with recombinant human TSH causes a global increase in thyroid uptake of 131I, and appears to improve the therapeutic outcome [42, 43]. 7.6.4 Summary Endemic goiter is the result of iodine deficiency, occasionally in association with goitrogens, with decrease in hormone production and compensatory increase in TSH secretion. Hyperfunctioning nodules may result from a growth advantage of some cells or gain-of-function mutations of the TSH receptor. Nodular thyroid disease is a common cause of subclinical hyperthyroidism in the elderly, and it may be associated with atrial fibrillation and osteopenia. Radionuclide studies typically show heterogeneous tracer distribution in the thyroid gland, with a normal or mildly elevated 24-h radioiodine uptake. Recombinant human TSH increases the thyroid uptake globally, and may facilitate the treatment of nodular goiters with 131I. 215 216 7 Thyroid Gland Fig. 7.2a–d. Scintigraphic images in four types of hyperthyroidism show: a multinodular goiter, b solitary hyperfunctioning thyroid nodule, c thyroiditis, d Graves’ disease 7.7 Destructive (“Subacute”) Thyroiditis 7.7.1 Postpartum Thyroiditis Destructive thyroiditis, also referred to as “subacute thyroiditis” or simply “thyroiditis”, is characterized by cell membrane breakdown and release of excessive amounts of thyroid hormone into the circulation. Serum thyroglobulin levels also are increased. The usual causes are autoimmune thyroid disease, viral infection, and amiodarone treatment. These are discussed below. Less commonly, thyroiditis may be related to treatment with interferon alpha, interleukin-2, lymphokine-activated killer (LAK) cells, and lithium. These therapeutic agents probably exacerbate existing autoimmune thyroid disease [73 – 77]. Bacterial thyroiditis is rarely encountered today. Thyroiditis tends to resolve spontaneously. Hyperthyroidism in the active phase is followed by transient hypothyroidism before restoration of the euthyroid state, usually in 6 – 12 months. Treatment usually consists of q -adrenergic blockers in the hyperthyroid phase, with analgesics for pain. Protracted thyroiditis may require glucocorticoids. Postpartum thyroiditis, also known as “painless” or “subacute lymphocytic” thyroiditis, is the principal thyroid disorder in postpartum women. It may be considered an accelerated form of autoimmune thyroid disease, attributed to suppression of immune-related disorders during pregnancy with a rebound after childbirth [78 – 82]. For the same reason, Graves’ disease also may occur in the postpartum period, though less frequently, and a strong association with insulin-dependent diabetes mellitus, an autoimmune condition, has been noted. Postpartum thyroiditis, like other forms of destructive thyroiditis, is a self-limited disease, but tends to reoccur in subsequent pregnancies. Permanent hypothyroidism occurs in 20% – 25% of patients over a period of 5 years. The incidence is greater in iodine-replete regions with a higher prevalence of autoimmune thyroid disease. Elevated thyroid peroxidase (“anti-microsomal”) antibodies during pregnancy are associated with a sharp increase in postpartum thyroiditis. 7.8 Autoimmune Thyroid Disease 7.7.2 Viral Thyroiditis 7.7.4 Radionuclide Procedures Viral subacute thyroiditis, also known as “de Quervain’s” thyroiditis, usually occurs after an upper respiratory tract infection. The disorder tends to be seasonal and may occur in clusters, occasionally causing mini epidemics [83]. It usually presents as a painful and tender goiter, associated with general malaise and possibly fever. Inflammation frequently begins in one lobe of the thyroid and gradually spreads to involve the entire gland. Permanent hypothyroidism is uncommon. Poor radioiodine/99mTc-pertechnetate uptake in the thyroid gland is the hallmark of subacute thyroiditis of any etiology (Fig. 7.2). Decreased tracer uptake is related to TSH suppression by excessive thyroid hormone released from damaged follicles, and to decreased hormone synthesis in the damaged gland. The thyroid uptake and scan normalize with resolution of thyroiditis. The nuclear study is frequently used in hyperthyroid individuals to differentiate thyroiditis, with low uptake, from Graves’ disease, with high uptake. A thyroid uptake/scan also may be worthwhile in amiodarone-related hyperthyroidism, which may be due to jodbasedow or thyroiditis. A suppressed thyroid uptake is non-diagnostic, while a normal or high uptake suggests that jodbasedow is likely. The thyroid uptake measurement also helps determine the feasibility of 131I treatment in refractory cases. 7.7.3 Thyroiditis and Other Effects of Amiodarone Amiodarone is an iodine-rich benzofuran derivative used to treat and prevent cardiac arrhythmias. It may precipitate a number of thyroid conditions including thyroiditis, which appears to be related to a cytotoxic effect [55, 56]. Since amiodarone and its metabolite desethylamiodarone have long half-lives of up to 100 days, the thyroid-related effects can be protracted and occasionally may begin after stopping the drug. Amiodarone-induced thyroiditis generally requires treatment with a glucocorticoid. Permanent hypothyroidism is uncommon. Other side effects of amiodarone stem from its high iodine content (see Sect. 7.5, “Iodine Intake and Thyroid Function”). Thyroid hormone synthesis may increase or decrease. Increased hormone synthesis (jodbasedow) typically occurs in nodular thyroid glands, which are common in iodine-deficient areas. Decreased hormone synthesis, resulting from a persistent Wolff-Chaikoff effect, is more frequent in iodine-sufficient regions with a higher incidence of autoimmune thyroid disease. Treatment of amiodarone-induced hyperthyroidism depends on the cause, although this may be difficult to determine. Thyroiditis, as noted earlier, responds to glucocorticoid therapy. Jodbasedow is treated with a thionamide, and if needed with potassium perchlorate to deplete thyroid iodine content. A clear distinction between thyroiditis and jodbasedow is frequently not possible, and treatment should be initiated with both a glucocorticoid and a thionamide. In resistant cases, 131I treatment may be feasible if the radioiodine uptake is adequate. Thyroidectomy may be an alternative in refractory cases, or when continued amiodarone treatment and prompt relief of hyperthyroidism are required. Other actions of amiodarone are worth noting. It blocks peripheral conversion of T4 to T3, binding of T3 to its receptors, and thyroid release of T3 and T4. These effects may permit the use of amiodarone in very selected cases of hyperthyroidism [84]. 7.7.5 Summary Subacute thyroiditis is usually caused by exacerbation of autoimmune disease, viral infection, and amiodarone therapy. It is characterized by an initial thyroid-destructive phase, with release of stored hormone into the circulation. Nuclear studies in this phase show poor radiotracer uptake, and help differentiate thyroiditis from other causes of hyperthyroidism. The disorder is self-limited and treated symptomatically, though amiodarone-related thyroiditis tends to last longer and generally requires a glucocorticoid. Amiodarone may be associated with other thyroid disorders related to its high iodine content, including jodbasedow (iodine-induced hyperthyroidism) and hypothyroidism. 7.8 Autoimmune Thyroid Disease 7.8.1 Etiological Factors Autoimmune thyroid disease comprises two major entities, Hashimoto’s disease (also known as chronic autoimmune thyroiditis) and Graves’ disease. Variants of Hashimoto’s disease include “subacute” thyroiditis, which occurs typically in the postpartum period, and atrophic thyroiditis. There is a genetic predisposition to the disease, with contribution from environmental factors [65, 85 – 89]. As discussed earlier, iodine excess has been associated with autoimmune thyroid disease. Cigarette smoking has been linked to exacerbation of autoimmune thyroid conditions including Graves’ ophthalmopathy, and increased occurrence in women im- 217 218 7 Thyroid Gland plies a role of sex steroids. The relationship between psychological stress and Graves’ disease presumably is related to immune suppression and rebound. A similar mechanism is believed to apply to postpartum thyroid dysfunction. The occasional occurrence of Graves’ disease in couples suggests that infection may be a precipitating factor. In support of this hypothesis, antibodies to certain microbial proteins have been found to crossreact with the human TSH receptor. Rarely, Graves’ disease may be precipitated by 131I treatment of nodular goiter in patients with underlying autoimmune thyroid disease [90]. Follicular disruption and release of thyroid antigens is believed to be the initiating event in these instances. Onset of Graves’ disease after subacute thyroiditis probably represents an analogous situation [91, 92]. 7.8.2 Pathophysiology Elevation of thyroid peroxidase antibodies is characteristic of Hashimoto’s disease. Antithyroglobulin antibodies also may be elevated. Hormone synthesis is impaired with compensatory increase in TSH secretion, which stimulates thyroid function and growth. Eventually, many patients become hypothyroid. Both overt and subclinical hypothyroidism related to autoimmune disease are widely prevalent in iodine-sufficient regions [93 – 95]. Exacerbation of Hashimoto’s disease, frequently occurring in the postpartum period, is a cause of subacute thyroiditis (see “Postpartum Thyroiditis”). Graves’ disease is associated with high levels of thyrotropin receptor autoantibodies (TRAB) that stimulate thyroid growth, and thyroid hormone synthesis and release [86 – 89]. Most organ systems are affected by Graves’ disease, the cardiovascular manifestations being the most apparent [31 – 33]. Increased heart rate and contractility increases the cardiac output. These effects are related to a direct inotropic effect of T3, decreased systemic vascular resistance, increased preload related to a higher blood volume, and heightened sensitivity to sympathetic stimulation. Blood volume is increased by activation of the renin-angiotensin-aldosterone system caused by the reduction in systemic vascular resistance, and by increased erythropoietin activity. Overt cardiac failure may result from severe and prolonged hyperthyroidism, but is rarely seen today. Atrial fibrillation is not an uncommon complication, occurring in up to 15% of patients with hyperthyroidism. 7.8.3 Radionuclide Procedures Nuclear studies are non-specific in Hashimoto’s disease. The thyroid gland is usually symmetrically en- larged with uniform tracer distribution, and the 24-h uptake is normal. In subacute thyroiditis resulting from exacerbation of Hashimoto’s disease, tracer uptake is typically absent or very low. Graves’ disease typically shows uniformly increased tracer uptake in a diffusely enlarged thyroid gland, frequently with visualization of a pyramidal lobe (Fig. 7.2). However, atypical appearances, particularly in Graves’ disease superimposed on nodular goiter, are occasionally encountered. If needed, TRAB measurement may assist in confirming the diagnosis. The 24-h uptake is elevated and, on average, much higher than in toxic nodular goiter. 131I treatment and antithyroid drugs remain the primary means of management of Graves’ disease. 7.8.4 Summary Autoimmune thyroid disorders, including Hashimoto’s disease and Graves’ disease, are related primarily to genetic susceptibility, with contributions from environmental factors including chronic iodine excess. Elevated serum anti-TPO antibodies are characteristic of Hashimoto’s disease. Exacerbation of Hashimoto’s disease, usually observed in postpartum women, may cause subacute thyroiditis with hyperthyroidism. Scintigraphy in such cases shows poor tracer uptake in the thyroid gland. Graves’ disease is characterized by elevated TSH receptor antibodies (TRAB). It affects most organ systems, but the cardiovascular manifestations generally are the most pronounced, and cardiac complications are not uncommon. The thyroid uptake and scan may be used to confirm the diagnosis of Graves’ disease and differentiate it from a destructive thyroiditis. 7.9 Thyroid Dysfunction During Gestation 7.9.1 Hyperthyroidism Hyperthyroidism during pregnancy is usually caused by gestational transient thyrotoxicosis (GTT) or Graves’ disease [48, 49]. Gestational transient thyrotoxicosis appears to be related to the TSH-like effects of human chorionic gonadotropin (hCG), which increases in early gestation. The condition resolves spontaneously in the second half of pregnancy. The incidence and severity of GTT are variable. It is occasionally associated with hyperemesis gravidarum. As in other hyperthyroid conditions, the serum TSH is low and free T4 may be elevated, but thyroid autoantibodies including TSH receptor antibodies (TRAB) are absent, since GTT is not an autoimmune condition. References Graves’ disease in pregnancy is a more serious condition associated with significant maternal and fetal risks, including pre-eclampsia, premature delivery, low infant birth weight, neonatal Graves’ disease, and central congenital hypothyroidism [48, 49, 96]. Characteristically, TRAB levels are elevated. Management of gestational Graves’ disease poses several challenges. Iodine-131 therapy is contraindicated, and thyroidectomy is inherently risky for both the mother and the fetus. Left untreated or inadequately treated, Graves’ disease in pregnancy increases the risk of fetal hyperthyroidism because of the transplacental passage of maternal TRAB. Of the available treatment options, thionamides – either PTU or methimazole – appear to be the safest. These drugs help control hyperthyroidism and reduce TRAB levels, but should be used only in small doses since they cross the placenta and may decrease fetal thyroid function [15, 49]. Graves’ disease tends to improve in the later stages of pregnancy, probably due to immune suppression, allowing thionamides to be tapered or discontinued. But therapy should be resumed after childbirth because of the risk of recurrence related to postpartum immune rebound. 7.9.2 Hypothyroidism Normal neurological development is dependent on adequate maternal and fetal thyroid function, and on thyroid hormone sufficiency in the early neonatal period [45 – 50, 97]. Iodine deficiency, present in regions of endemic goiter, may be associated with hypothyroidism in both the mother and the fetus, and may cause varying severities of neurological and growth retardation including cretinism. Fortunately, the incidence of these disorders has declined due to iodine supplementation programs. Maternal thyroid hormone is increasingly recognized as an important factor in fetal development in the second and third trimesters. Maternal hypothyroidism alone, i.e., without fetal hypothyroidism, has been linked to neuropsychological deficits in the offspring, and to increased risk of fetal loss and preterm delivery. Autoimmune thyroid disease is the most frequent cause of hypothyroidism in the mother. While overt iodine deficiency is relatively uncommon today, iodine intake has gradually declined in many “iodine-sufficient” areas and may actually fall short of requirement during pregnancy. This may have the potential to aggravate autoimmune hypothyroidism. 7.9.3 Summary Hyperthyroidism in pregnancy is generally caused by GTT or Graves’ disease. Management of Graves’ disease remains a challenge, with thionamide treatment as the best option. Patients should be monitored closely because undertreatment, with persistently high maternal TRAB, increases the risk of fetal hyperthyroidism, while overtreatment may cause fetal hypothyroidism. Gestational hypothyroidism is usually related to autoimmune thyroid disease, and less frequently to iodine deficiency. The latter may be associated with fetal hypothyroidism as well. Neurological development is influenced by maternal thyroid function, fetal thyroid function, and thyroid hormone levels in the newborn. References 1. Pankow BG, Michalak J, McGee MK (1985) Adult human thyroid weight. Health Physics 49:1097 – 1103 2. Mochizuki Y, Mowafy R, Pasternack B (1963) Weights of human thyroids in New York City. Health Physics 9: 1299 – 1301 3. Wolff J (1964) Transport of iodide and other anions in the thyroid gland. Physiol Rev 44:45 4. De la Vieja A, Dohan O, Levy O, Carrasco N (2000) Molecular analysis of the sodium/iodide symporter: Impact on thyroid and extrathyroid pathophysiology. Physiol Rev 80:1083 – 1105 5. Alexander C, Bader JB, Shaefer A, et al (1998) Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 39:1551 – 1554 6. Kappes RS, Sarkar SD, Har-El G, et al (1994) Iodine-131 therapy of thyroid cancer: extensive contamination of the hospital room in a patient with tracheostomy. J Nucl Med 35:2053 – 2054 7. Mitchell G, Pratt BE, Vini L, et al (2000) False positive 131I whole body scans in thyroid cancer. Br J Radiol 73:627 – 635 8. Romney B, Nickoloff EL, Esser PD (1989) Excretion of radioiodine in breast milk. J Nucl Med 30:124 – 126 9. Stoffer SS, Hamburger JI (1975) Inadvertent I-131 therapy for hyperthyroidism in the first trimester of pregnancy. J Nucl Med 17:146 – 149 10. Roti E, Minelli R, Gardini E, et al (1994) The iodine perchlorate discharge test before and after one year of methimazole treatment of hyperthyroid Graves’ disease. J Clin Endocrinol Metab 78:795 – 799 11. Bijayeswar V, Coffey R, Coyle R et al (1999) Concurrence of Pendred syndrome, autoimmune thyroiditis, and simple goiter in one family. J Clin Endocrinol Metab 84:2736 – 2738 12. Frills J (1987) The perchlorate discharge test with and without supplement of potassium iodide. J Endocrinol Invest 10:581 – 584 13. Sarkar SD, Savitch I (2004) Management of thyroid cancer. Applied Radiol November:34 – 45 14. Sarkar SD (1996) Thyroid pathophysiology. In: Sandler MP, Coleman RE, Th. Wackers FJ (eds) Diagnostic nuclear medicine. Williams and Wilkins, Baltimore, pp 899 – 909 15. Cooper DS (2005) Antithyroid drugs. N Engl J Med 352:905 – 917 16. Schneider AB, Becker DV, Robbins J (2002) Protecting the thyroid from accidental or terrorist-instigated 131I releases. Thyroid 12:271 – 272 17. Koong S, Reynolds JC, Movius EG et al (1999) Lithium as a potential adjuvant to I-131 therapy of metastatic, well-differentiated thyroid carcinoma. J Clin Endocrinol Metab 84:912 – 916 219 220 7 Thyroid Gland 18. Sarkar SD (2006) Benign thyroid disease, in lifelong learning and self-assessment program (LLSAP), Society of Nuclear Medicine (www.snm.org) 19. Sarkar SD, Becker DV (1995) Thyroid uptake and imaging. In: Becker KL (ed) Principles and practice of endocrinology and metabolism. Lippincott, Philadelphia, pp 307 – 313 20. Sarkar SD, Kalapparambath T, Palestro CJ (2002) Comparison of I-123 and I-131 for whole body imaging in thyroid cancer. J Nucl Med 43:632 – 634 21. Casara D, Rubello D, Saladini G, et al (1993) Different features of pulmonary metastases in differentiated thyroid cancer: Natural history and multivariate statistical analysis of prognostic variables. J Nucl Med 34:1626 – 1631 22. Ward LS, Santarosa PL, Granja F, et al (2003) Low expression of sodium iodide symporter identifies aggressive thyroid tumors. Cancer Lett 200:85 – 91 23. Hooft L, Hoekstra OS, Deville W, et al (2001) Diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in the follow-up of papillary or follicular thyroid cancer. J Clin Endocrinol Metab 86:3779 – 3786 24. Wang W, Larson SM, Fazzari M, et al (2000) Prognostic value of 18F-fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab 85:1107 – 1113 25. Chin BB, Patel P, Cohade C, et al (2004) Recombinant human thyrotropin stimulation of fluoro-D-glucose positron emission tomography in well-differentiated thyroid carcinoma. J Clin Endocrinol Metab 89:91 – 95 26. Moog F, Linke R, Manthey N, et al (2000) Influence of thyroid stimulating hormone levels on uptake of FDG in recurrent and metastatic differentiated thyroid carcinoma. J Nucl Med 41:1989 – 1995 27. Freitas J, Gross MD, Sarkar SD (2003) Laboratory (in vitro) assessment of thyroid function. In: Sandler MP et al (eds) Diagnostic nuclear medicine, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 591 – 606 28. Prummel MF, Brokken LJS, Wiersinga WM (2004) Ultra short-loop feedback control of thyrotropin secretion. Thyroid 14:825 – 829 29. Refetoff S, Weiss RE, Usala SJ (1993) The syndromes of resistance to thyroid hormone. Endocr Rev 14:348 – 399 30. Burmeister LA, Goumaz MO, Mariash CN, Oppenheimer JH (1992) Levothyroxine dose requirements for thyrotropin suppression in the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 75:344 – 350 31. Klein I, Ojamaa K (2001) Thyroid hormone and the cardiovascular system. N Engl J Med 344:501 – 509 32. Kahaly GJ, Dillmann WH (2005) Thyroid hormone action in the heart. Endocrine Rev, July, e-publication 33. Parle JV, Maisonneuve P, Sheppard MC, et al (2001) Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 358:861 – 865 34. Bauer DC, Ettinger B, Nevitt MC, et al (2001) Risk for fracture in women with low serum levels of thyroid-stimulating-hormone. Ann Intern Med 134:561 – 568 35. Hartoft-Nielsen M-L, Lange M, Rasmussen AK, et al (2004) Thyrotropin-releasing hormone stimulation test in patients with pituitary pathology. Horm Res 61:53 – 57 36. Sarkar SD, Torres MA, Manalili E et al (1998) Iodine-131 effects on thyroid remnant function: influence of serum TSH levels. Radiology 209P:404 37. Ladenson PW, Braverman LE, Mazzaferri EL et al (1997) Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N Engl J Med 337:888 – 896 38. Haugen BR, Pacini F, Reiners C, et al (1999) A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 84:3877 – 3885 39. Mazzaferri EL, Robbins RJ, Spencer CA, et al (2003) A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 88:1433 – 1441 40. Sarkar SD, Afriyie MO, Palestro CJ (2001) Recombinant human thyroid-stimulating-hormone-aided scintigraphy: Comparison of imaging at multiple times after I-131 administration. Clin Nucl Med 26:392 – 395 41. Rudavsky AZ, Freeman LM (1997) Treatment of scannegative, thyroglobulin-positive metastatic thyroid cancer using radioiodine I-131 and recombinant human thyroid stimulating hormone. J Clin Endocrinol Metab 82:11 – 14 42. Huysmans DA, Nieuwlaat W-A, Hermus AR (2004) Towards larger volume reduction of nodular goitres by radioiodine therapy: a role for pretreatment with recombinant human thyrotropin? Clin Endocrinol 60:297 – 299 43. Albino CC, Junior M, Olandoski M, et al (2005) Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab 90:2775 – 2780 44. Dunn JT (2002) Guarding our nation’s thyroid health. J Clin Endocrinol Metab 87:486 – 488 45. Aghini-Lombardi F, Antonangeli L, Martino E et al (1999) The spectrum of thyroid disorders in an iodine-deficient community. The Pescopagano Survey. J Clin Endocrinol Metab 84:561 – 566 46. Boyages SC (1993) Iodine deficiency disorders. J Clin Endocrinol Metab 77:587 – 591 47. Haddow JE, Palomaki GE, Allan WC et al (1999) Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549 – 555 48. Lazarus JH (2002) Epidemiology and prevention of thyroid disease in pregnancy. Thyroid 12:861 – 865 49. Glinoer D (2003) Management of hypo- and hyperthyroidism during pregnancy. Growth Horm IGF Res 13 Suppl A:S45 – 54 50. American Academy of Pediatrics (1993) Newborn screening for congenital hypothyroidism: recommended guidelines. Pediatrics 91:1203 – 120 51. Fradkin JE, Wolff J (1983) Iodide-induced thyrotoxicosis. Medicine 62:1 – 20 52. Wartofsky L, Ransil BJ, Ingbar SH (1970) Inhibition by iodine of the release of thyroxine from the thyroid glands of patients with thyrotoxicosis. J Clin Invest 49:78 – 86 53. Pedersen IB, Knudsen N, Jorgensen T, et al (2002) Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: A prospective comparative register-based population survey. J Clin Endocrinol Metab 87:4462 – 4469 54. Laurberg P, Pedersen KM, Hreidarsson A et al (1998) Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab 83:765 55. Bogazzi F, Bartalena L, Gasperi M, et al (2001) The various effects of amiodarone on thyroid function. Thyroid 11: 511 – 519 56. Daniels GH (2001) Amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab 86:3 – 8 57. Livadas DP, Koutras DA, Souvatzoglou A, et al (1977) The toxic effects of small iodine supplements in patients with autonomous thyroid nodules. Clin Endocrinol 7:121 – 127 References 58. Laurie AJ, Lyon SG, Lasser EC (1992) Contrast material iodides: potential effects on radioactive iodine thyroid uptake. J Nucl Med 33:237 – 238 59. Konno N, Makita H, Yuri K et al (1994) Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab 78:393 – 397 60. Beierwaltes WH (1969) Iodine and lymphocytic thyroiditis. Bull All-India Institute Med Sci 3:145 61. Bagchi N, Sundick RS, Hu LH et al (1996) Distinct regions of thyroglobulin control the proliferation and suppression of thyroid-specific lymphocytes in obese strain chickens. Endocrinology 137:3286 – 3290 62. Chandra AK, Mukhopadhyay S, Lahari D, et al (2004) Goitrogenic content of Indian cyanogenic plant foods & their in vitro anti-thyroidal activity. Indian J Med Res 119:180 – 185 63. Elnour A, Hambraeus L, Eltom M, et al (2000) Endemic goiter with iodine sufficiency: a possible role for the consumption of pearl millet in the etiology of endemic goiter. Am J Clin Nutr 71:59 – 66 64. Hershman JM (2005) Perchlorate and thyroid function: What are the environmental issues? Thyroid 15:427 – 431 65. Vestergaard P, Rejnmark L, Weeke J, et al (2002) Smoking as a risk factor for Graves’ disease, toxic nodular goiter, and autoimmune hypothyroidism. Thyroid 12:69 – 75 66. Curran PG, DeGroot LJ (1991) The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr Rev 12:135 – 150 67. Gaitan E (1988) Goitrogens. Ballieres Clin Endocrinol Metab 2:683 – 702 68. Studer H (1989) Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev 10:125 – 135 69. O’Sullivan C, Barton CM, Staddon SL, et al (1991) Activating point mutation of the gsp oncogene in human thyroid adenomas. Mol Carcinog 4:345 – 349 70. Tonacchera M, Chiovato L, Pinchera A et al (1998) Hyperfunctioning thyroid nodules in toxic multinodular goiter share activating thyrotropin receptor mutations with solitary toxic adenoma. J Clin Endocrinol Metab 83:492 – 498 71. Krohn K, Fuhrer D, Bayer Y, et al (2005) Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev 26:504 – 524 72. Castro MR, Gharib H (2005) Continuing controversies in the management of thyroid nodules. Ann Intern Med 142:926 – 931 73. Atkins MB, Mier JW, Parkinson DR et al (1988) Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N Engl J Med 318:1557 – 1563 74. Vialettes B, Guillerand MA, Viens P, et al (1993) Incidence rate and risk factors for thyroid dysfunction during recombinant interleukin-2 therapy in advanced malignancies. Acta Endocrinol 129:31 – 38 75. Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F et al (1998) Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med 158:1445 – 1448 76. Mazziotti G, Sorvillo F, Stornaiuolo G, et al (2002) Temporal relationship between the appearance of thyroid autoantibodies and development of destructive thyroiditis in patients undergoing treatment with two different type-1 interferons for HCV-related chronic hepatitis: A prospective study. J Endocrinol Invest 25:624 – 630 77. Dang AH, Hershman JM (2002) Lithium-associated thyroiditis. Endocr Pract 8:232 – 236 78. Amino N, Tada H, Hidaka Y et al (1999) Screening for postpartum thyroiditis. J Clin Endocrinol Metab 84:1813 79. Stagnaro-Green A (2002) Postpartum thyroiditis. J Clin Endocrinol Metab 87:4042 – 4047 80. Premawardhana LDKE, Parkes AB, John R, et al (2004) Thyroid peroxidase antibodies in early pregnancy: Utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid 14:610 – 615 81. Parker RH, Beierwaltes WH (1961) Thyroid antibodies during pregnancy and in the newborn. J Clin Endocrinol Metab 21:792 82. Sarlis NJ, Brucker-Davis F, Swift JP, et al (1997) Graves’ disease following thyrotoxic painless thyroiditis. Analysis of antibody activities against the thyrotropin receptor in two cases. Thyroid 7:829 – 836 83. De Bruin TWA, Riekhoff FPM, de Boer JJ (1990) An outbreak of thyrotoxicosis due to atypical subacute thyroiditis. J Clin Endocrinol Metab 70:396 – 402 84. Brusco F, Gonzalez G, Soto N, et al (2004) Successful treatment of hyperthyroidism with amiodarone in a patient with propylthiouracil-induced acute hepatic failure. Thyroid 14:862 – 865 85. Fountoulakis S, Tsatsoulis A (2004) On the pathogenesis of autoimmune thyroid disease: a unifying hypothesis. Clin Endocrinol 60:397 – 409 86. Weetman AP (2000) Graves’ disease. N Engl J Med 343: 1236 – 1248 87. Rees-Smith B, Bolton J, Young S, et al (2004) A new assay for thyrotropin receptor autoantibodies. Thyroid 14:830 – 835 88. Rees-Smith B, McLachlan SM, Furmaniak J (1988) Autoantibodies to the thyrotropin receptor. Endocr Rev 9:106 – 121 89. Gupta M (1992) Thyrotropin receptor antibodies: advances and importance of detection techniques in thyroid disease. Clin Biochem 25:193 – 199 90. Nygaard B, Knudsen JH, Hegedus L et al (1997) Thyrotropin receptor antibodies and Graves’ disease, a side-effect of I-131 treatment in patients with nontoxic goiter. J Clin Endocrinol Metab 82:2926 – 2930 91. Wartofsky L, Schaaf M (1987) Graves’ disease with thyrotoxicosis following subacute thyroiditis. Am J Med 83:761 – 764 92. Litaka M, Morgenthaler NG, Momotani N, et al (2004) Stimulation of thyroid-stimulating hormone (TSH) receptor antibody production following painless thyroiditis. Clin Endocrinol 60:49 – 53 93. Sawin CT, Castelli WP, Hershman JM (1985) The aging thyroid: thyroid deficiency in the Framingham study. Arch Intern Med 145:1386 – 1388 94. Surks MI, Ortiz E, Daniels GH, et al (2004) Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228 – 238 95. Cooper DS (2004) Subclinical thyroid disease: consensus or conundrum? Clin Endocrinol 60:410 – 412 96. Kempers MJE, van Tijn DA, van Trotsenburg ASP, et al (2003) Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab 88:5851 – 5857 97. Stagnaro-Green A, Chen X, Bogden JD, et al (2005) The thyroid and pregnancy: A novel risk factor for very preterm delivery. Thyroid 15:351 – 357 221