* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Solution 22. - Tutor Breeze
Survey
Document related concepts
Transcript
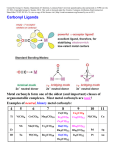
NCERT/CBSE CHEMISTRY CLASS 12 textbook http://www.TutorBreeze.com Contact for Online Tutoring in Physics, Math, Chemistry, English, Accounts, MBA Solutions/Answers to NCERT/CBSE CHEMISTRY Class 12(Class XII)textbook CHAPTER NINE Coordination Compounds 9.22 Discuss the nature of bonding in metal carbonyls. Metal carbonyls are coordination complexes of transition metals with carbon monoxide. These complexes contain CO ligands, such as nickel carbonyl (Ni(CO)4). Carbon monoxide bonds to transition metals using pi* back-bonding." A sigma bond arises from overlap of nonbonding electron pair on carbon with d, s, and p-orbitals on the metal. A pair of π bonds arises from overlap of filled d-orbitals on the metal with a pair of π-antibonding orbitals projecting from the carbon of the CO. This electron donation makes the metal more electron rich, and in order to compensate for this increased electron density, a filled metal d-orbital may interact with the empty pi* orbital on the carbonyl ligand to relieve itself of the added electron density. This second component is called pi-backbonding or pi.. We can describe the bonding of CO to a metal as consisting of two components. The first component is a two electron donation of the lone pair on carbon into a vacant metal d-orbital. This electron donation makes the metal more electron rich, and in order to compensate for this increased electron density, a filled metal d-orbital may interact with the empty pi* orbital on the carbonyl ligand to relieve itself of the added electron density. This second component is called pibackbonding or pi-backdonation. ©TutorBreeze.com Please do not copy the answer given here Write to us for help in understanding the solution
![[1] Conduction electrons in a metal with a uniform static... A uniform static electric field E is established in a...](http://s1.studyres.com/store/data/008947248_1-1c8e2434c537d6185e605db2fc82d95a-150x150.png)

![[E]ven the most difficult problems in chemical experimentation can](http://s1.studyres.com/store/data/006510251_1-96239c5b6e245cee1be60ed8e0730b48-150x150.png)