* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 3-4 Energy - Bakersfield College
100% renewable energy wikipedia , lookup
Regenerative brake wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Energy subsidies wikipedia , lookup
Kinetic energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Internal energy wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
World energy consumption wikipedia , lookup
Alternative energy wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy policy of Australia wikipedia , lookup
Negawatt power wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Distributed generation wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
United States energy law wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Conservation of energy wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
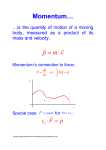
Chapter 3-4a Energy 3-1. The Meaning of Work 3-2. Power 3-3. Kinetic Energy 3-4. Potential Energy 3-5. Energy Transformations 3-6. Conservation of Energy 3-7. The Nature of Heat 3-8. Linear Momentum 3-9. Rockets 3-10. Angular Momentum 3.11 Special Relativity 3.12 Rest Energy 3.13 General Relativity 3-1. Work Work equals force times distance. W = Fd The SI unit of work is the joule. 1 joule (J) = 1 newton-meter (N · m) W=Fd=(100N)(8m)=800N·m=800J 3-2. Power Power is the rate at which work is being done: P = W/t SI unit of power is the watt. 1 watt (W) = 1 joule/second (J/s) The kilowatt (kW) is a convenient unit of power for many applications. Horsepower • James Watt – Perfected the steam engine 200 years ago – Had to provide a comparison to the work output of a horse. He found that: • Typical horse could perform 497 W of work for as much as 10 hours a day • Watt increased the standard to 746 W – 1 horsepower (hp) = 746 W = 0.746 kW – 1 kilowatt (kW) = 1.34 hp – Early steam engines ranged from 4-100 hp 3-3. Kinetic Energy Telekinesis Video 1 Telekinesis Video 2 Energy is that property something has that enables it to do work. The energy of a moving object is called kinetic energy (KE): KE = ½mv2 where m = mass and v = speed. KE increases very rapidly with speed because of the v2 factor. 3-4. Potential Energy Potential energy (PE) is the energy an object has by virtue of its position. Gravitational Potential Energy: PE = mgh 3-5. Energy Transformations Energy can be transformed or converted from one form to another. 3-5. Energy Transformations Types of Energy 1. Kinetic energy 2. Potential energy 3. Chemical energy 4. Heat energy 5. Electric energy 6. Radiant energy 3-6. Conservation of Energy The law of conservation of energy states that energy cannot be created or destroyed, although it can be changed from one form to another. Matter can be considered as a form of energy; matter can be transformed into energy and energy into matter according to the law of conservation of energy. Eo = moc2 where Eo = rest energy, mo = rest mass, and c = speed of light (3x108m/s or 186,000 miles/sec). Bakersfield College Physical Science PHSC-B11 Count Rumford 3-7. Nature of Heat American (Benjamin Thompson) Supported British during Revolutionary War Count Rumford the the Movedsupported to Britain after war andBritish changed hisin name to Count Revolutionary WarRumford and supervised the Supervised making cannons making ofcannons. He observed that Observedprocess heat given offheat during boring during the boring was process given off (frictional(frictional heat)heat) that could be used to Heat could bebe usedproduced to boil water, and could be boil water and could over and produced again from the same piece of metal over again from the same piece of metal. Conclusion: Heat must be a form of energy Heat mustbe energy. 3-8. Linear Momentum Linear Momentum is a measure of the tendency of a moving object to continue in motion along a straight line: p = mv 3-8. Linear Momentum The law of conservation of momentum states: In the absence of outside forces, the total momentum of a set of objects remains the same no matter how the objects interact with one another. 3-8. Linear Momentum Newton’s Cradle-an example of the conservation of linear momentum. 3-9. Rockets The momentum of the exhaust gases is balanced by the rocket's upward momentum. Multistage rockets are more efficient than single-stage, and so are widely used. 3-9. Rockets Rockets are a version of Newton’s third law of motion as well as the conservation of linear momentum. 3-10. Angular Momentum Angular momentum is a measure of the tendency of a rotating object to continue spinning about a fixed axis L=mvr L= angular Momentum m=mass circling v=velocity of rotation r=distance from center The smaller the “r” the faster the “v”. Angular momentum is conserved. 3-10. Angular Momentum • Definition: – The more angular momentum an object has, the greater its tendency to continue to spin (and be stable) • Toy tops • The earth • Footballs • Bullets – Defining angular momentum is complicated; depends on… • How fast the object is turning • Mass of the object • How the mass is distributed (the further the mass is from the center of the object, the greater the angular momentum) 3-10. Angular Momentum Gyroscopes The Segway Due to angular momentum, when a force is applied in one direction, the combined forces, including the angular momentum, will be in a perpendicular direction. http://www.youtube.com/ watch?v=GeyDf4ooPdo 3-10. Angular Momentum Naval Gyroscopes used to stabilize ships and guns Naval Ship Stabilization Naval Gyroscopes used to stabilize ships and guns 3-11. Special Relativity Albert Einstein (1879-1955) published the special theory of relativity in 1905. Special relativity is based on two postulates: 1. The laws of physics are the same in all frames of reference moving at constant velocity. 2. The speed of light (c ) in free space has the same value for all observers (c = 3 x 108 m/s) 3-11. Special Relativity mo = m γ heavier to = t / γ slower lo = l / γ shorter •• Twin Paradox Muon Experiment 3-11. Special Relativity Twin Paradox http://www.youtube. com/watch?v=qgCNDpt-mw http://www.youtube.c om/watch?v=DWKn _Punrjk http://www.youtube.com/ watch?v=gdRmCqylsME Muon Experiment 3.12 Rest Energy 3.12 Rest Energy • E = mc2 or Energy and Mass are the same! • Example 3.8 p 91 – How much mass is converted to energy in a 100MW nuclear power plant? T=(60)(60)(24)= 86,400 s/day E=Pt=108W(86,400 s/day)=8.64 x 1012J m = E/c2 = 8.64 x 1012J/(3 x 108m/s)2 m = 9.6 x 10-5kg or about 0.000013 oz 3-13. General Relativity General theory of relativity was developed by Einstein in 1916, which related gravitation to the structure of space and time and showed that even light was subject to gravity. Chapter 4 Energy 4-2 Energy Consumption 4.3 Global Warming 4.4 Greenhouse Effect 4.5 Liquid Fuels 4.6 Natural Gas 4.7 Coal 4.8 A Nuclear World 4.13 The Future 4.1 The Energy Problem 1. Oil and natural gas reserves will last about another century.. 2. Although coal reserves will last several hundred more years, mining coal is dangerous, and burning coal creates environmental problems such as acid rain, air pollution, and enhanced global warming. 3. The potential for a large-scale nuclear accident is present. 4. Discharge of radioactive wastes into the environment from badly run nuclear power plants has occurred. 5. An unsolved disposal problem of radioactive nuclear waste exists. 4.2 Energy Consumption Energy consumption 2003 Fig.4.5 4.3 Global Warming • Greenhouse Effect 31 4.3 Global Warming • Atmospheric CO2 Controlled by water cycle Could increase temperature by 10oC 32 4.3 Global Warming 4.3 Global Warming 4.3 Global Warming 4.3 Global Warming 4.3 Global Warming 4.3 Global Warming Use of Various Fuels 4.5 Liquid Fuels Petroleum, a mixture of various hydrocarbons, is the source of most liquid fuels. 4.5 Hydroelectric Energy 4.6 Gas Fuels Natural gas is largely methane, CH4. Syngas Coal can be gasified 4.6 Natural Gas 4.7 Solid Fuels Types of solid fuels include coal, wood, and coke 4.7 Coal 4.7 Solid Fuels Acid rain from sulfur impurities in coal. 4.8 A Nuclear World Chernobyl Nuclear Accident 4.8 A Nuclear World Chernobyl Nuclear Accident http://www.ems.psu.edu/~radovic/Chernobyl.html 4.8 A Nuclear World 4-13. The Future Fig. 3.42 Fig. 3.39 Fig. 3.40 Fig. 3.41 4-13. The Future-Algae Farms to Produce Biofuels 4-13. The Future-Algae Farms to Produce Biofuels