* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download courtship and mating behavior as a reproductive isolating
Survey
Document related concepts
Transcript
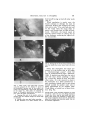
AM. ZOOLOGIST, 4:147-153(1964). COURTSHIP AND MATING BEHAVIOR AS A REPRODUCTIVE ISOLATING MECHANISM IN DROSOPHILA LEE EHRMAN The Rockefeller Institute, Neiu York Species of sexually reproducing or* ganisms are genetically closed systems. They are closed systems because they do not exchange genes or do so rarely enough so that the species differences are not swamped. Races are, on the contrary, genetically open systems. They do exchange genes by peripheral gene flow, unless they are isolated by extrinsic causes such as spatial separation. The biological meaning of the closure of a genetic system is simple but important—it is evolutionary independence. Consider these four species—man, chimpanzee, gorilla, orangutan. No mutation and no gene combination arising in any one of them, no matter how favorable, can benefit any of the others. It cannot do so for the simple reason that no gene can be transferred from the gene pool of one species to that of another. On the contrary, races composing a species are not independent in their evolution; a favorable genetic change arising in one race is, at least potentially, capable of becoming a genetic characteristic of the species as a whole. Species are genetically closed systems because the gene exchange between them is impeded or prevented by reproductive isolating mechanisms. The term "isolating mechanism" was proposed by Dobzhansky in 1937 as a common name for all genetically conditioned barriers to gene exchange between sexually reproducing populations. According to Mayr (1963), isolating mechanisms are ". . . perhaps the most important set of attributes a species has. . . ." It is a remarkable fact that isolating mechanisms are physiologically and ecologically a most heterogenous collection of phenomena. It is another remarkable fact that the The work reported here has been carried out under Contract No. AT-(30-l)-3096, U. S. Atomic Energy Commission. isolating mechanisms which maintain the genetic separateness of species are quite different not only in different groups of organisms but even between different pairs of species in the same genus. Several classifications of the reproductive isolating mechanisms have been proposed. That of Mayr is a simple and convenient one. The two major groups are the premating barriers which prevent the formation of hybrid zygotes, and the postmating barriers which impede the survival or reproduction of these zygotes. Three of the premating mechanisms are: 1) Potential mates do not meet (seasonal and habitat isolation). 2) Potential mates meet but do not mate (ethological or sexual isolation). 3) Copulation attempted but no transfer of gametes takes place (mechanical isolation). Four of the postmating barriers are: 4) Gametes transferred but no fertilization, and hence no zygote formation takes place (gamete mortality). 5) Death of the zygotes (hybrid inviability). 6) The Fi zygotes are viable but partly or completely sterile (hybrid sterility). 7) Fi hybrids are fertile but the fitness of the F 2 or backcross hybrid is reduced (hybrid breakdown). Since our primary interest today centers on the relations between genes and behavior, ethological isolation, sometimes also termed sexual or psychological isolation, should be discussed here in more detail. The phenomenon observed is usually that the mutual attraction between conspecific females and males is greater than the attraction between males and females of different species. Successful analysis of this form of isolation requires (147) 148 LEE EHRMAN a detailed description of the courtship rituals and the mating behavior in the pure species concerned. Species of the genus Drosophila offer abundant and favorable material for such analysis; their courtship and mating can be observed easily at any season of the year and under reasonably precisely controlled laboratory conditions. My favorite materials are the incipient species of the Drosophila paulistorum complex or superspecies. As the name "incipient species" implies, these forms are very closely related and can be regarded either as very similar sibling species or as very distinct races standing on the brink of full species separation. This close relationship is, of course, very favorable for analytical purposes. What we wish to study is speciation in the process and the closeness permits a genetic analysis to be carried further than would be possible with full species. The six incipient species of Drosophila paulistorum inhabit a part of the Neotropical zoogeographic region, from Guatemala and Trinidad in the north to southern Brazil in the south. Each incipient species has a distribution area of its own, but these are in part overlapping, so that in some places two, three, or even four incipient species live together, sympatrically, and apparently without producing any hybrids. Thanks to the work of many authors, especially H. T. Spieth (1952) the sexual behavior in Drosophila is fairly well known. Sexual recognition is a trial and error affair among drosophilids. Males will generally attempt to court females of any Drosophila species, even distantly related ones. At least four distinct elements can be distinguished in the courtship process in Drosophila paulistorum. The first element or stage is that of circling. The male approaches a female, attracts her attention, and may limit her movements by running around her. He never completes a full circle however, turns around, reversing the direction of his movement about every 330°. Next he begins a tapping action—an important aspect of the courtship in this species (Fig. 1A) . The male lightly touches the legs or the abdomen of the female with his own legs. At first, only one leg of each fly is involved. Since many taps may be necessary before it is established that the male has located a female of his own species, the male continues to circle between contacts. Here, the question of the female's receptivity is settled (this is probably mediated by samples taken by the great number of chemoreceptive hairs on the body of both insects); she will either remain still so that circling is no longer necessary, and then spread her wings to receive the male between them; or she will vigorously kick the courting male and do her best to depart. Licking and wing vibrating occur next (Fig. IB), both as a prelude to mounting. As Spieth (1952) has described it, the male "goes to the rear of the female and assumes a slightly crouched position with the tip of his abdomen slightly curled. Having positioned himself, he extends one wing 70° to 90° and vibrates it periodically, twisting his body on the longitudinal axis as he vibrates, taps (uppercuts), and occasionally licks or attempts to lick the female." In licking, the male proboscis contacts the female genitalia—and this is a reliable sign that the male is about to rush in for the mount. In D. paulistorum wing vibrations are not so important a part of courtship as in some of the other species. The one wing that is vibrated is used for leverage as the male raises himself on the body of the female. Mounting and insertion seem to be accomplished simultaneously. A portion of the male reproductive organ is used in a clasping manner and, after mounting, the male secures his position on the female by placing his forelegs on top of her slightlyspread wings. This additional support is imperative because copulation lasts a fairly long time in this species (an average of 17 minutes and 12 seconds). During the copulatory period, the female may turn about or walk around; and she may even BEHAVIORAL ISOLATION IN Drosophila 149 find herself having to fend off other males (Fig- 2) • When copulation is nearly over, the female attempts to dislodge the male by vigorously kicking and swinging her body from side to side. The male loses his hold on her wings when she snaps them together, and seconds later he falls off backwards. Thereafter, the female repels all sexually excited males by raising the tip of her abdomen, rendering her vaginal orifice inaccessible. FIG. 2. Photograph of the full mount during which time the female may move about freely and even feed. FIG. 1. These "stills" were taken from a 16 mm color film prepared by Mr. Richard F. Carter of the Rockefeller Institute and by the author; the project was supported by a grant from The Society of the Sigma Xi. This study of courtship be havior in Drosophila paulistorum was shown, in part, when this paper was delivered. A. Tapping (the male is the smaller, active individual with the rumpled wings)—an initial stage in courtship. B. Licking—often seen just before mounting. C. The formation of a chain wherein one re- Notice that throughout this entire procedure, it is the female that at all stages, is "discriminatingly passive" while the male is "indiscriminately eager" (Bateman, 1948). D. paulistorum males are very active and will court almost anything: a dead fly, a mired fly, lumps of food, and often other males. It is not unusual to observe the formation of a chain initiated by a male courting a female, and in turn being himself courted by another male who is being approached by yet another male. These chains, of course, are of short duration (Fig. 1C). jecting female (with extruded ovipositor) is being courted by a male who is in turn being courted by a male, etc., for a total of one female and three males—these chains are necessarily of short duration but do emphasize the fact that D. paulistorum males will certainly court the other males. See text for detailed explanation. 150 LEE EHRMAN Now that the normal courtship and mating behavior of the species-complex has been recorded, we are ready to consider the evolution of this superspecies Drosophila paulistorum (Dobzhansky et al., 1964). One of the first of the many interesting evolutionary phenomena exhibited by the D. paulistorum complex of seven known races or incipient species to be analyzed genetically was the complete hybrid male sterility discovered when crosses between the races were successful. The male sterility was found to be genie in nature (Ehrman, 1960a), and to be expressed via the genotype of hybrid mothers. This kind of hybrid sterility, unique in the genetic literature, operates in the following manner. Suppose that we cross two races, A and B, one or both of them having one or more chromosomes marked with suitable mutant genes. The distribution in the progeny of the chromosomes of different racial origin can then be followed by inspection of the external morphology of the Hies. The interracial hybrid males are sterile, but the females are fertile and can be backcrossed to either A or to B males. After several backcrosses, flies are obtained which carry all but one of the chromosomes from race A, or all but one from race B. Females of this sort are, of course, fertile and can again be backcrossed to males of the recurrent race. Half of this progeny, of either sex, will carry all chromosomes of the same race, and half will contain one race-foreign chromosome. (Crossingover is suppressed in these hybrids.) Now, the striking fact is that males of both kinds are completely sterile. The sterility of the males identical in chromosomal constitution with males of one of the "pure" races can only mean that the sterility is in this case determined not by the chromosomal constitution of the individual itself but by that of his mother. The presence of at least one race-foreign chromosome in the developing egg cell before meiosis somehow modifies the cytoplasm or some other property of the egg, and makes a male individual developing from this egg sterile. Once the above facts were established, the question logically arose whether the ethological isolation observed in races of D. paulistorum has the same peculiar genetic basis as the sterility of the hybrid males, that is, a genic-maternal effect. The ethological isolation is in all probability the mechanism which keeps the gene pools of these races separate in nature where the races share the same territory. Indeed, race hybrids have never been found in nature, and the cytological study of their chromosomes by Dobzhansky and Pavlovsky (1962) shows that genetically effective hybridization of the races is rare, if it occurs at all. The genetic basis of the ethological (sexual) isolation was studied by Ehrman (1961). The method used was in principle the same as that applied for the analysis of the hybrid sterility, i.e., making crosses and backcrosses of strains of different races having some of their chromosomes marked with suitable mutant genes. It was here that the knowledge of the courtship and mating behavior of D. paulistorum became important. Hybrid males of D. paulistorum transfer no sperm into the body of the female with which they copulate. F1 hybrid males produce no motile spermatozoa, because a restitution nucleus is formed presumably after the first meiotic division, and then all the gametic material degenerates. Backcross hybrid males are even more profoundly sterile, since they frequently have no testes at all, or only one testis, or no gonial cells within the abnormally thick testicular sheath. Yet these males are normal in external morphology and have normal genitalia and internal reproductive organs other than testes. Their patterns of courtship and mating behavior are also normal. Since dissections of the female reproductive tract for the storage of transferred sperm (as is usually done in analyses of this sort where fertile males are involved) would be out of the question here; the entire analysis of the genetic architec- BEHAVIORAL ISOLATION IN ture of sexual isolation as a reproductive isolating mechanism was made by using the simple but informative, direct-observation technique. One may conclude from these directobservation studies that the sexual isolation, which makes matings between the females and males of the D. paidistonim races much less likely to occur than matings within the races, is due to polygenes in every one of the three pairs of chromosomes which the species possesses. These polygenes control the sexual preferences of their carriers. Their effects seem to be simply additive. A female of hybrid origin which carries a majority of the chromosomal material derived from a given race is most likely to accept a male of that race. And conversely, a hybrid male is most likely to be accepted by females whose chromosomal constitution is closest to his. The source of the cytoplasm or the genetic constitution of the mother do not seem to matter. This is clearly not at all comparable with the genetic basis of the hybrid sterility, where the properties of an egg are determined, as far as the sterility of the backcross males is concerned, by the genotype present in it before meiosis, and not by that formed following fertilization. We may surmise that this peculiar chromosomal-cytoplasmic mechanism of sterility arose first in the evolutionary history of these races, and that it arose in allopatric races becoming adapted to different environments in their respective geographic areas. Sexual isolation might then be built by natural selection, as a much less wasteful and more efficient reinforcement of the bar to gene exchange between the races. When this sexual isolation became strong or complete, the races became able to coexist sympatrically, as they have been found to do in some localities in the northern part of the South American continent (Dobzhansky et al, 1964) where as many as four races have been found to live in the same places. How effective can sexual isolation be, what with omnipresent variations in environmental influences and the undeniable Drosophila 151 susceptibility of behavior patterns to these influences? Consider the case of the rare hybrids obtained in the laboratory between the races of Drosophila paulistorum inhabiting northern and southern Brazil, respectively. These hybrid individuals are most difficult to obtain and possess a genetic constitution discordant enough so that the hybrid females repel the courtship of all males, and will mate with none; their sterile hybrid brothers will court and will be rejected by almost all females, including their own hybrid siblings. There is no question of gene flow through the hybrid males, because they are absolutely sterile. Their sisters, however, are potentially fertile. This has been verified not only by dissection of their internal and external reproductive apparatus, but more conclusively by a direct experiment. Some etherized hybrid females are exposed to many mature, unanesthetized males; the males approach, mount and inseminate the females in question; the hybrid females subsequently recover and deposit fertilized eggs which develop normally (Ehrman, 1960b). More recently, Carmody et al. (1962) investigated a possible correlation between the occurrence of hybrid sterility and sexual isolation within the D. paidistonim complex. In this massive study, more than sixteen thousand females from all of the then known D. paulistorum races were dissected and their sperm-storing organs checked. Each Drosophila paulistorum female, hybrid or not, has three sperm-storing organs. The "male choice" experimental method was used to test mating preferences of different strains comprising the races. Briefly, this involves groups of ten virgin females of each of two races ( a total of twenty females), aged for three to four days after hatching, marked for recognition by clipping a very small part of one of the wings of one of two types of females, and then confined with males of one of the two races for twenty-four to fortyeight hours, i.e., A ^ X A ?? -)- B J J or B ^ X B $? + A 5$. All the females are then dissected in a physiological saline, 152 LEE EHRMAN and their sperm receptacles are examined for the presence or absence of sperm. Strains of the same race but of different geographic origin often show significant preferences for homogamic matings, but strains of different races show such preferences to a much greater extent. On the average, sexual isolation is lower in interracial matings involving the transitional race, the bridge to fertile hybrids between all the other races. The degree of sexual isolation shows only a weak positive correlation with the fertility or sterility of the hybrids between the strains crossed. More study is needed to unravel the interrelations of the sexual isolation and hybrid sterility. As indicated above, sexual isolation is most effective in limiting or preventing the appearance of interracial hybrids with reduced reproductive fitness. A problem of considerable interest is whether isolating mechanisms which decrease the chances of production of hybrid offspring are stronger between sympatric than between allopatric populations of the same pairs of races or subspecies or incipient species. When two or more Mendelian populations of sexually reproducing and cross-fertilizing organisms share the same territory, these populations are exposed to the risk of hybridization and gene exchange. If such a gene exchange leads to production of adaptively inferior genotypes, natural selection may favor genetic constitutions which hinder or prevent hybridization. On the other hand, the gene exchange and introgression may weaken the reproductive isolation, and may eventually lead to fusion of the previously separate populations. Experiments were made to test whether the sexual isolation between sympatric strains of a given pair of races is, on the average, greater or smaller than that between allopatric strains of the same races. They showed (Dobzhansky et al., 1964) that natural selection had encouraged the spread and maintenance, in sympatric populations of incipient species, of those genes which limit or prevent the reproductive wastage resulting from gene ilow between these populations. However, one may suppose as Muller (1942) does that reproductive isolation arises as an incidental by-product of genetic divergence. When populations become adapted to different environments they are likely to become different in progressively more and more genes. Reproductive isolation then might arise because the action of many genes is pleiotropic. Some gene differences selected for different reasons, or resulting from random genetic drift, may thus have isolating sideeffects. That selection can indeed produce, or at least strengthen, reproductive isolation has been demonstrated experimentally by Koopman (1950) and by Knight, Robertson, and Waddington (1956). Koopman set up experimental populations in laboratory population cages consisting of two species, D. pseudoobscura and D. persimilis. Each species was homozygous for a different recessive mutant gene with easily visible external effects. The pure species and the hybrids were thus made easily distinguishable. In every generation the adult flies were taken from the cages, etherized, classified, and counted. The hybrids were then discarded and new population cages were started with nonhybrid progenies. By these means Koopman was selecting the progenies of intraspecific, and excluding those of interspecific, matings. In a surprisingly small number of generations he obtained strains of D. pseudoobscura and D. persimilis which showed a more nearly complete sexual isolation than did the original strains. The results of Knight, Robertson, and Waddington are, in a way, even more dramatic, since they obtained by selection a significant, though of course incomplete, sexual isolation of strains of D. melanogaster which originally showed no such isolation. The incipient species of D. paulistorum seem to furnish a good illustration of the two processes postulated above. It is not easy to imagine the sterility of male hybrids between these incipient species conferring am adapthe advantage upon them. But once this sterility has arisen as a hy- BEHAVIORAL ISOLATION IN product of their genetic divergence, it is probable that natural selection would favor genetic constitutions which make the sterile hybrids rare. The sexual isolation between the incipient species may be a reproductive barrier contrived as such by the action of natural selection. Dobzhansky and Spassky (1959) first suggested that Drosophila paulistorum is a cluster of species in statu nascendi, a borderline case of uncompleted speciation. This suggestion is borne out by the work since then. Drosophila paulistorum is a superspecies composed of six races or incipient species. The interest of the situation lies precisely in that these six may be considered about equally legitimately as very distinct races or as very closely related species. Each race inhabits a geographic area different from the others, but the areas of some of the races overlap. Where two or more "races" share a common territory they apparently do not interbreed, and thus behave like full-fledged species. The Transitional race and transitional strains yield, however, fertile hybrids with some other races. The possibility of gene flow between the incipient species is, therefore not excluded, although it is questionable whether it is actually taking place. Last year, in his Vice Presidential address on Genes and the Study of Behavior, Caspari (1963) cautioned ". . . the analysis of Ft species hybrids cannot give us, in principle, information about genetic units. This will only be possible through further crosses, F2 and backcrosses." The step-bystep, often tedious analysis of the D. paulistorum situation, as presented here today, certainly indicates how correct he was. Understanding of the genetic basis of the sexual isolation among these races would have been impossible without a systematic study of the backcross individuals, in conjunction with a survey of the courtship and mating behavior in the Fj hybrids and in the Drosophila 153 "pure" races. Surely "the genetic basis of behavioral characters is important, because it is the genes which are the basic units transmitted and reshuffled in the evolutionary process and which are arranged by selective forces into adaptive action patterns." (Caspari, 1963.) REFERENCES Bateman, A. J. 1948. Intra-sexual selection in Drosophila. Heredity 2:349-368. Carmody, G., A. Diaz Collazo, Th. Dobzhansky, L. Ehrraan, J. S. Jaffrey, S. Kimball, S. Obrebski, S. Silagi, J. T. Tidwell, and R. Ullrich. 1962. Mating preferences and sexual isolation within and between the incipient species o£ Drosophila paulistorum. Am. Midi. Nat. 68:67-82. Caspari, E. 1963. Genes and the study oC behavior. Am. Zoologist 3:97-100. Dobzhansky, Th. 1937. Genetics and the origin of species. First edition. Columbia Univer. Press, New York. Dobzhansky, Th., L. Ehrman, O. Pavlovsky, and B. Spassky. 1964. The superspecies Drosophila paulistorum. Proc. Natl. Acad. Sci. 51:3-9. Dobzhansky, Th., and O. Pavlovsky. 1962. A comparative study of the chromosomes in the incipient species of the Drosophila paulistorum complex. Chromosoma 13:196-218. Dobzhansky, Th., and B. Spassky. 1959. Drosophila paulistorum, a cluster of species in statu nascendi. Proc. Natl. Acad. Sci. 45:419-428. Ehrman, L. 1960a. The genetics of hybrid sterility in Drosophila paulistorum. Evolution 14:212-223. . 1960b. A genetic constitution frustrating the sexual drive in Drosophila paulistorum. Science 131:1381-1382. -. 1961. The genetics of sexual isolation in Drosophila paulistorum. Genetics 46:1025-1038. Knight, G. R., A. Robertson, and C. H. Waddington. 1956. Selection for sexual isolation within a species. Evolution 10:14-22. Koopman, K. F. 1950. Natural selection for reproductive isolation between Drosophila pseudoobscura and Drosophila persimilis. Evolution 4: 135-148. Mayr, E. 1963. Animal species and evolution. Harvard Univer. Press, Cambridge, Mass. Muller, H. J. 1942. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6:71-125. Spieth, H. T. 1952. Mating behavior within the genus Drosophila (Diptera). Bull. Am. Mus. Nat. Hist. 99:395-474.