* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aspirin and Antiinflammatory Agents
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Psychopharmacology wikipedia , lookup
Cell encapsulation wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Hyaluronic acid wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
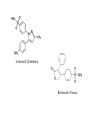
Aspirin and Antiinflammatory Agents Chapter 17 History of Aspirin • Hippocrates (~ 460 - 377 B.C.): historical records of pain relief treatments, including powder made from willow tree bark, leaves to help heal headaches, pains and fevers • The Royal Society of London publishes an article by the Rev. Edward Stone, "Account of the success of the Bark of the Willow in the Cure of Agues," officially reporting what had been folklore for centuries • 1826: Brugnatelli and Fontana (Italians) obtained salicin in impure form • 1828: Johann Buchner, University of Munich professor of pharmacy, isolated tiny amount of bitter tasting yellow, needle-like crystals, which he called salicin from willow bark • 1829: Henri Leroux (French chemist) improved extraction procedure 30g from 1.5kg of bark • 1838: Raffaele Piria (Italian chemist) purified salicylic acid – BUT salicylic acid “tough on stomachs” so searched for 'buffering‘ • 1853: Charles Frederic Gerhardt (French chemist) neutralized salicylic acid by buffering w/ sodium (sodium salicylate) and acetyl chloride acetylsalicylic acid – Worked, but Gerhardt did not market it and abandoned his discovery • 1899: Felix Hoffmann (German chemist; worked for Bayer) rediscovered Gerhardt's formula – Admin’d to his father who was suffering from arthritis w/ good results – Convinced Bayer to market the new wonder drug – Aspirin patented March 6, 1889 • “Aspirin”: “A" in acetyl chloride, "spir" in spiraea ulmaria (plant from which salicylic acid derived), “in” familiar name ending for medicines – Aspirin ® and Heroin ® were once trademarks belonging to Bayer • Aspirin first sold as powder – First Aspirin tablets made in 1915 • After Germany lost World War I, Bayer forced to give up both trademarks as part of the Treaty of Versailles in 1919 – Reduces "aspirin" to generic word for any brand of acetylsalicylic acid • 1950: Dr. Lawrence L. Craven (US) describes aspirin's action as a blood-thinner, begins prescribing daily doses to his patients as a means of preventing heart attacks • 1971: John R. Vane (British pharmacologist) discovers aspirin's mechanism of action —inhibits prod’n prostaglandins -- hormone-like substances in the body – 1982: Sir John R. Vane is co-winner of the Nobel Prize in Medicine for his discoveries concerning prostaglandins • 1990s: Studies show regular use of aspirin may reduce risk of colon cancer. • 2005: Research shows aspirin reduces risk of stroke in healthy women, although no clear benefit is seen for prevention of heart attack Acute Inflammatory Rxn • Response of mammalian host to invading pathogen or noxious agent – If deficient, suppressed opportunistic infections – If inappropriate autoimmune dysfunctions • Many, varied biochem mediators – Interactions w/ each other, immune response biochem’s/cells Eicosanoids • Generated de novo from membr phospholipids – From esterified fa • Eicosa=20 C; tetraenoic=4 db’s • History: 1930s – substance in semen uterine contractions – Believed originated in prostate (so prostaglandin) • Now recognize family of mol’s in most tissues, der’d from arachidonic acid • Biosynthesis – PLA2 cleaves arachidonic acid from membr phospholipid • Also cleaves lyso-PAF – precursor of another mediation of inflamm’n (PAF) Rang 15.6 • Not stored, but synth’d when needed – Stimuli for PLA2 activity vary w/ tissue • Ag-Ab rxns on mast cells • Bradykinin binding on fibroblasts • Thrombin binding on platelets – Free fa further metab’d by • Fatty acid cylooxygenases 1 and 2 • Lipoxygenases • CYPs • Primarily locally-active – Commonly work at cell/tissue/structure from which synth’d (autocoids) – Blood concent very low • Efficient pulmonary degradation Brody 17-1 flowchart Cyclooxygenase (COX) • Two forms: COX-1, COX-2 – Exploited by drug designers • COX-1 – Constitutive enz (always present) in most cells – “Housekeeping protein” – Prostanoids prod’d impt to normal homeostasis (ex: regulation vascular responses) • COX-2 – Induced in inflammatory cells by inflammation stimuli – Inhibited by NSAIDs • Arach acid further metab’d differently in diff cells – Platelets TXA2 – Vasc endothelium, macrophages PGI2 – Most impt: PGE2, PGI2, PGD2, PGF2a, TXA2 Prostanoid Receptors • Five main classes • Typical G-protein coupled receptors – – – – – DP-receptors FPIPTPEP-, based on 5 classes prostanoids + TXA2 • Modulate adenylyl cyclase – Stimulators: DP, EP2, EP4, IP – Inhibitors: EP2 • Modulate phosphlipase C DAG/IP3 and Ca+2 mobilization • Many cells have >1 PG receptor subtype • Eicosanoids do not enter cells except w/ transport system intake – Lung, renal prox tubules, thyroid plexus, ciliary body Actions of Prostanoids • PGD2 vasodilation, inhib’n platelet aggreg’n, relaxation gi muscle, uterine relaxation, mod’n release hypothl/ pituitary hormones • PGF2a contraction myometrium (humans); luteolysis (cattle); vasoconstriction (dogs, cats) • PGI2 vasodilation; inhib’n platelet aggreg’n • TXA2 vasoconstriction; platelet aggreg’n • PGE2 contraction bronchial & gi smooth muscle (EP1 receptor); relaxation bronchial, vascular, gi smooth muscle (EP2); contract’n intest smooth muscle and pregnant human uterus, inhib’n gastric acid secr’n, inhib’n lipolysis and autonomic neurotransmitter release (EP3) Prostanoids and Inflamm’n • PGE2 -- Predom prostanoid w/ inflamm response; PGI2 also generated – Prod’d by local tissues, blood vessels w/ acute inflamm’n • PGD2 released by mast cells – With chronic inflamm’n, PGE2 and TXA2 released by monocytes/macrophages • Powerful vasodilators – Synergize w/ histamine, bradykinin • Redness w/ inflamm’n due to dilation precapillary arterioles by prostaglandins – Incr’d blood flow – Histamine + bradykinin also required • Sensitize afferent neurons to bradykinin pain • PGE’s impt to fever – Found in high concent in csf – Prod’d in hypothalamus in response to pyrogen (IL1) rel’d by bacteria – Elevation temp set-point Leukotrienes • Prod’d from membr phospholipids by 5lipoxygenase – Adds hydroperoxy grp to C5 of arach acid ( HPETE) • Further metab LTA4 LT’s B4-F4 – LTC4,D4,E4 = Slow Reacting Substance of Anaphylaxis – “Cysteinyl-leukotrienes” Actions of Leukotrienes • LTB4 powerful chemotactic for neutrophils, macrophages – On neutrophils upreg’n membrane adhesion mol’s; incr’d prod toxic O2 prod’s; release granule enz’s – On macrophages stim’n prolif’n; cytokine release – Receptor of phosphatidylinositol/DAG type incr’d cell [Ca] • LTD4 impt to respiratory system – Spasmogens – Incr’d mucous secr’n – Red’d airway conductance • LTD4 impt to cardiovascular system – Decr bp (constrict’n small coronary vessels) – Wheal/flare w/ subcu dose • LTB4 impt to bronchial hyperreactivity in asthmatics; role in cardiovascular changes w/ acute anaphylaxis Drugs Inhibiting Prostanoid Prod’n • Two main types – Glucocorticoids – Non-steroidal antiinflammatories (NSAIDs) • NSAIDs – Used worldwide • > 50 on market • Many have unwanted effects – Three major types of effects • Mod’n inflammatory rxn (antiinflamm) • Red’n pain (analgesic) • Lower body temp (antipyretic) Commonly Used NSAIDs • • • • • • • Aspirin Ibuprofen Diflunisal Fenbufen Diclofenac Mefenamic Acid Nabumetone • • • • • • • Acetaminophen Naproxen Sulindac Indomethacin Tolmetin Piroxicam Tenoxicam ibuprofen Fenbufen Sulindac Indomethacin • Most traditional NSAIDs have sim antiinflammatory activity except – Indomethacin, piroxicam may be stronger – Aspirin has diff pharmacological actions • Antipyresis activity relieves fever • Analgesia effective against arthritis, bursitis, muscular/vascular pain, toothache, dysmenorrhea, postpartum pain – Headache pain relieved by blocking cerebral vascular dilation w/ prostanoid decr NSAID Mechanism of Action: COX Inhib’n • COX enz’s bifunctional – Main activity PGG2 – Peroxidase activity converts PGG2PGH2 • Inhibitors block only main rxn • COX enz’s assoc’d w/ cell membr – Active site hydrophobic, accepts arachidonic acid – Rxn: insertion 2 O, extraction free radical 5C ring COX-1 vs COX-2 • COX-2 active site slightly wider • Aa523 differs – COX-1 has leucine • Rel bulky – COX-2 has valine • Smaller; leaves gap • Allows access to side-pocket – COX-2 selective agents have side chain, interacts w/ pocket – May be too large to fit COX-1 active site channel Celecoxib (Celebrex) Rofecoxib (Vioxx) • Traditional NSAIDs – H-bond polar arginine (120) half-way down channel blockage of channel • COX-1 inhib’n instantaneous, competitively reversible • COX-2 inhib’n incr’s w/ time – Also reversible (by competitively excluding arach acid) • Aspirin – Binds, acetylates serine530 irrev inact’n of both enz’s • NSAIDs may inhibit inflammation by other mech’s also – Some scavenge oxygen radiacls prod’d by neutrophils, macrophages (ex: sulindac) – Aspirin inhbits expr’n transcr’n factor NF-kB • Impt to transcr’n genes for mediators of inflamm’n COX-1 vs COX-2 • COX-2 most responsible for prod’n prostanoids impt to inflammation – COX-2 inhib’n predicts best antiinflamm response • Most NSAIDs inhibit both COX enz’s (diff extent inhib’n) – COX-1 inhib’n predicts unwanted gi tract sideeffects (irritation) • Selective COX-2 inhibitors marketed • Celecoxib (“Drug Side Effect Lawyers”; incr’d risk heart attack) • Rofecoxib (Vioxx withdrawal) NSAID Adverse Reactions • Numerous; may cause death • Elderly w/ joint diseases need fairly large doses, long-continued use – High incidence side effects: gi, liver, skin, kidney, spleen, blood, bone marrow • Gastrointestinal disturbances commonest – Due to COX-1 inhib’n • Impt for PGS that inhibit acid secr’n, protective of mucosa • Side-effects include dyspepsia, diarrhea (or constipation), nausea, vomiting, gastric bleeding, ulceration – May hemorrhage, perforation – Can admin PGs to relieve – Selective COX-2 inhib’s decr’d gi effects • Skin Reactions – Second most common side-effect – Mild rashes, urticaria, photosensitivity • May be fatal – Most frequently w/ mefenamic acid, sulindac • Renal Effects – Some pts susceptible • Reversible w/ stopping drug – PGs synth’d here impt to vasodilation at kidney w/ angiotensin II, noradrenaline – Can chronic nephritis, renal papillary necrosis – Impt w/ paracetamol (now withdrawn) Aspirin • Acetylsalicylic acid • Among most commonly consumed world-wide • Rel insoluble; Na+ and Ca+2 salts readily soluble • Many effects beyond antiinflammatory – Antiplatelet for cardiovascular disorders – Decr’d colon, rectal cancer – Decr’d risk, later onset of Alzheimer’s • Weak acid, unionized in stomach – good abs’n – Most abs’n in ileium (more surface area) • Metab by ox’n (25%); glucuronide or sulfate conjugation (50%); excr’d unchanged (25%) – Rate excr’n incr’d in alkaline urine • Irreversible inhibitor of COX enz’s • Toxicity may be local or systemic – Same side-effects as NSAIDs – Salicylism: tinnitis, vertigo, decr’d hearing w/ large, repeated doses – Reye’s syndrome in children: liver and CNS disturbances – May alter acid-base balanceby uncoupling ox’ve phosph’n incr’d blood [O2] alteration breathing resp alkalosis Acetaminophen • One of most common non-narcotic analgesic/antipyretic – Rel weak (?) antiinflammatory activity – Selective for COX-3 (recently described) • Given orally; well absorbed – Peak plasma concent’s 30-60 min’s • Plasma ½ life 2-4 h – Glucuronidated or sulfated in liver • Unwanted effects few at therapeutic doses – Large doses over long period increases renal damage risk • Toxic doses (2-3x max therapeutic) hepatotoxicity – Potentially fatal – Phase II enz’s sat’d products of mfo’s (Phase I enz’s) in incr’d concent • N-acetyl-p-benzoquinone imine – Usually metab’d by conjugation glutathione – Depletion glutathione suff imine to react w/ cellular nucleophiles necrosis liver, kidney tubules • Init symptoms poisoning nausea, vomiting • Hepatotoxicity occurs 24-48 h later • Treatment – Gastric lavage, then – Oral activated charcoal – If early, acetylcysteine IV or methionine orally incr’d glutathione in liver enhanced metab/excr’n Drugs that Inhibit Leukotriene Synthesis/Activity • Zileuton – inhibits 5-lipoxygenase – Antiasthmatic – Zyflo • Zarfirlukast, Montelukast – Cys-LT receptor antagonists – Antiasthmatic – Accolate, Singulair (Zyflo) Montelukast (Singulair) Zafirlukast (Accolate) Glucocorticoids • For antiinflammatory activity, work through both innate and adaptive responses – Through induction/inhib’n transcription of modulator proteins • Innate via cyclooxygenase modulation, leukocyte mediators • Adaptive via cytokines and pathogen-assoc’d prot’s Innate Responses via Leukocytes • Mediators gen’d from both cells and plasma – Modify, regulate vascular and cellular events • Tissue macrophages recognize pathogenassoc’d molec patterns on invading microorganisms – Interaction triggers release cytokines (esp IL1, TNF-a, chemokines) • IL-1, TNF-a vasc dilation, fluid exudation – Exudate has enz cascade mol’s kinin system, complement system • release histamine from mast cells local dilation arterioles • W/ local tissue damage cytokines rel’d eicosanoids synth’d (PGI2, PGE2 vasodilation, leukotrienes chemotaxis prot’s) • Expression of adhesion mol’s on cell surfaces – Draw leukocytes toward pathogen phagocytosis Adaptive Responses via Leukocytes • Lymphocytes: T cells, B cells • Cloned for specific attack on partic invader – Humoral response via B cells, Ab’s – Cell mediated response via T cells • Produce various prot’s to modulate, coordinate responses of other leukocytes (innate and adaptive) Endogenous Glucocorticoids • Steroids secr’d by adrenal cortex • Synth’d, rel’d w/ ACTH from ant pit – ACTH secr’n regulated • Corticotropin Releasing Factor from hypothal • Blood [glucocorticoid] – CRF secr’n regulated • Blood [glucocorticoid] • CNS input – Opioid peptides inhibitory – Psych factors inhibitory or stimulatory – Injury, infection • Basal glucocorticoids in blood – Highest 8 a.m. – Lowest midnight • Metabolic actions – Carbohydrate: decr’d uptake, utilization glu; incr’d gluconeogenesis; hyperglycemia – Proteins: incr’d catab; decr’d anab – Fat: permissive lipolysis; fat redist’n • Regulation of inflammatory response Mechanism of Antiinflammatory Action • Glucocorticoid receptors cytoplasmic – – – – Steroid hormones lipophilic Control gene transcr’n Found in most cells 3000 to 10000 per cell, depending on tissue • Receptor binding conform’l change exposure DNA-binding domain and dimerization • Bound dimer nucleus • Bind steroid response elements on DNA • Repression or induction partic genes Glucocorticoids Repress Transcr’n • Through inhib’n transcr’n factors AP-1, NF-kB • Repression genes for COX-2 – So no prostanoids, leukotrienes • Repression genes for cytokines, adhesion factors – So lessens macrophage activity • Others Glucocorticoids Induce Transcr’n • Annexin-1 (= lipocortin-1) – Impt to neg faeedback control at hypothal/ant pit – Antiinflammatory (inhibits PLA2?) • Others • Take sev hours Antiinflammatory Actions of Glucocorticoids • Red’d vasodilation, decr’d fluid exudation • At acute inflamm’n decr’d influx, activity of leukocytes • At chronic inflamm’n decr’d activity macrophages, decr’d angiogenesis • Decr’d prod’n, action of cytokines (IL’s, TNF), eicosanoids, IgG, complement components • Overall: red’n chronic inflamm’n, autoimmune rxns BUT decr’d healing, protections of inflamm responses • Decr’d redness, heat, pain, swelling • Decr’d wound healing, repair • Regardless of cause of inflamm’n – Invaders, chem/phys stimuli, hypersensitivity/autoimmunity • Used to suppress graft rejection • May prevent “overshoot” of endogenous responses Unwanted Effects • Occur w/ large doses, prolonged admin • Suppression of response to infection, injury • Sudden withdrawal suppression ability synthesize endogenous hormones • Metabolic, water/electrolyte effects w/ endogenous hormones – Swelling, Cushing’s syndrome • Calcium, phosphate regulation by endogenous hormones – So osteoporosis • Metabolic effects of endogenous hormones – So growth inhib’n in children Pharmacokinetics • Various routes of admin – Active orally – IM/IV – Topically • Fewer side effects • Binding to corticosteroid-binding globulin and albumin – Bound forms inactive • Hydrocortisone ½ life 90 mins (main biol effects in 2-8 h) Clinical Uses • Replacement in adrenal failure (Addison’s disease) • Antiinflammatory for asthma; skin/ear/ eye inflamm’n; hypersensitivity disorders; autoimmune disorders; transplant pts • Neoplastic disease for red’n cerebral edema; in combination w/ cytotoxic drugs; antiemesis w/ chemotherapy Corticosteroid Agents • • • • • • • Hydrocortisone Cortisone Corticosterone Prednisolone Prednisone Methylprednisolone Triamcinolone • Dexamethasone • Betamethasone • Beclometasone dipropionate • Budesonide • Deoxycortone • Fludrocortisone • Aldosterone Hydrocortisone Corticosterone • http://opioids.com/heroin/heroinhistory. html • www.library.ucla.edu • inventors.about.com/library/inventors/bl aspirin.htm • http://www.intelihealth.com/IH/ihtIH/ WSIHW000/8124/23697/237089.html? d=dmtContent